Abstract
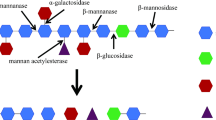
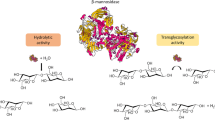
Mannan is an important polysaccharide found in softwoods and many other plant sources. Mannans from various sources display large differences in composition, structure and complexity. To hydrolyse mannan into its monomer sugars requires a number of enzymes working in synergy. This review examines mannan structure and the enzymes required for its hydrolysis. Several studies have investigated the effect of supplementing β-mannanases with β-mannosidases and α-galactosidases in binary and ternary combinations. Synergistic enhancement of hydrolysis has been found in some, but not all cases. In the case of mannosidases, they sometimes display an anti-synergistic effect with mannanases, most likely due to competition for binding sites. Most importantly, in the case of α-galactosidases, the same enzyme from different families display differences in synergistic interactions due to different specificities. An improved understanding of enzyme interactions will aid in achieving enhanced hydrolysis of mannans and higher sugar yields. This review highlights areas which require further research in order to gain a better understanding of mannan hydrolysis and utilisation. Such knowledge is very important as this can be used in the optimisation of commercial or purified enzyme mixtures to improve the economic viability of the conversion of high mannan-containing biomass such as softwoods into fermentable sugars for bioethanol production.



Similar content being viewed by others
References
Ademark P, Larsson M, Tjerneld F, Stålbrand H (2001) Multiple α-galactosidases from Aspergillus niger: purification, characterization and substrate specificities. Enzym Microb Technol 29:441–448
Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A (2010) Plant cell walls: from biochemistry to biology. Garland Science, New York
Banerjee G, Scott-Craig JS, Walton JD (2010) Improving enzymes for biomass conversion: a basic research perspective. Bioenergy Res 3:82–92
Beukes N, Pletschke BI (2011) Effect of alkaline pre-treatment on enzyme synergy for efficient hemicellulose hydrolysis in sugarcane bagasse. Bioresour Technol 102:5207–5213
Cerveró JM, Skovgaard PA, Felby C, Sørensen HR, Jørensen H (2010) Enzymatic hydrolysis and fermentation of palm kernel press cake for production of bioethanol. Enzym Microb Technol 46:177–184
Charrier M, Rouland C (2001) Mannan-degrading enzymes purified from the crop of brown garden snail Helix aspersa Muller (Gastropoda Pulmonata). J Exp Zool 290:125–135
Chauve M, Mathis H, Huc D, Casanave D, Monot F, Ferreira NL (2010) Comparative kinetic analysis of two fungal β-glucosidases. Biotechnol Biofuels 3(3). doi:10.1186/1754-6834-3-3
Clarke JH, Davidson JK, Rixon JE, Halstead JR, Fransen MP, Gilbert HJ, Hazlewood GP (2000) A comparison of enzyme-aided bleaching of softwood paper pulp using combinations of xylanase, mannanase and α-galactosidase. Appl Microb Biotechnol 53:661–667
Dhawan S, Kaur J (2007) Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol 27(4):197–216
Dias FMV, Vincent F, Pell G, Prates JA, Centeno MSJ, Tailford LE, Ferreira LMA, Fontes CMGA, Davies GJ, Gilbert HJ (2004) Insights into the molecular determinants of substrate specificity in glycoside hydrolase family 5 revealed by the crystal structure and kinetics of Cellvibrio Mixtus mannosidase 5A. J Biol Chem 279(24):25517–25526
Dotsenko GS, Semenova MV, Sinitsyna OA, Hinz SWA, Wery J, Zorov IN, Kondratieva EG, Sinitsyn AP (2012) Cloning, purification, and characterization of galactomannan-degrading enzymes from Myceliophthora thermophila. Biochem. doi:10.1134/S0006297912110090
Ghosh A, Luis AS, Brás JLA, Fontes CMA, Goyal A (2013) Thermostable recombinant β-(1 → 4)-mannanase from C. thermocellum: biochemical characterization and manno-oligosaccharides production. J Agric Food Chem 61:12333–12344
Gübitz GM, Hayn M, Sommerauer M, Steiner W (1996) Mannan-degrading enzymes from Sclerotium rolfsii: characterisation and synergism of two endo β-mannanases and β-mannosidase. Bioresour Technol 58:127–135
Halstead JR, Fransen MP, Eberhart RY, Park AJ, Gilbert HJ, Hazlewood GP (2000) α-Galactosidase A from Pseudomonas fluorescens subsp. cellulosa: cloning, high level expression and its role in galactomannan hydrolysis. FEMS Microbiol Lett 192:197–203
Han Y, Dodd D, Hespen CW, Ohene-Adjei S, Schroeder CM, Mackie RI, Cann IKO (2010) Comparative analyses of two thermophilic enzymes exhibiting both β-1,4 mannosidic and β-1,4 glucosidic cleavage activities from Caldanaerobius polysaccharolyticus. J Bacteriol 192(16):4111–4121
Hägglund P (2002) Mannan-hydrolysis by hemicellulases: enzyme-polysaccharide interaction of a modular β-mannanase. Ph.D. Thesis. Lund University, Sweden
Jian H, Zhu L, Zhang W, Sun D, Jiang J (2013) Enzymatic production and characterization of manno-oligosaccharides from Gleditsia sinesis galactomannan gum. Int J Biol Macromol 55:282–288
Jindou S, Karita S, Fujino T, Hayashi H, Kimura T, Sakka K, Ohmiya K (2002) α-Galactosidase Aga27A, an enzymatic component of the Clostridium josui cellulosome. J Bacteriol 184:600–604
Kim W, Kobayashi O, Kaneko S, Sakakibara Y, Park G, Kusakabe I, Tanaka H, Kobayashi H (2002) α-Galactosidase from cultured rice (Oryza sativa L. var. Nipponbare) cells. Phytochem 61:621–630
Klyosov AA, Dotsenko GS, Hinz SWA, Sinitsyn AP (2012) Structural features of β-(1 → 4)-D-galactomannans of plant origin as a probe for β-(1 → 4)-mannanase polymeric substrate specificity. Carbohydr Res 352:65–69
Kulminskaya AA, Eneiskaya EV, Isaea-Ivanona LS, Salvel’ev AN, Sidorenko IA, Shabalin KA, Golubev AM, Neustroev KN (1999) Enzymatic activity and β-galactomannan binding property of β-mannosidase from Trichoderm reesei. Enzym Microb Technol 25:372–377
Malgas S, van Dyk JS, Pletschke BI (2015) β-mannanase (Man26A) and α-galactosidase (Aga27A) synergism—a key factor for the hydrolysis of galactomannan substrates. Enzym Microb Technol 70:1–8
Margolles-Clark E, Tenkanen M, Luonteri E, Penttila M (1996) Three α-galactosidase genes of Trichoderma reesei cloned by expression in yeast. Eur J Biochem 240:104–111
Moreira LR, Filho EX (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Nascimento AS, Muniz JRC, Aparício R, Gobulev AM, Polikarpov I (2014) Insights into the structure and function of fungal β-mannosidases from glycoside hydrolase family 2 based on multiple crystal structures of the Trichoderma harzianum enzyme. FEBS J 281:4165–4178
Reid JSG, Edwards ME (1995) Galactomannans and other cell wall storage polysaccharides in seeds. In: Stephen AM, Phillips GO, Williams PA (eds) Food polysaccharides and their applications. CRC Press, Boca Raton, pp 155–186
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30(5):279–291
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6:219–228
Shi P, Yao G, Cao Y, Yang P, Yuan T, Huang H, Bai Y, Yao B (2011) Cloning and characterization of a new β-mannosidase from Streptomyces sp. S27. Enzym Microb Technol 49:277–283
Tailford LE, Money VA, Smith NL, Dumon C, Davies GJ, Gilbert HJ (2007) Mannose foraging by Bacteroides thetaiotaomicron: structure and specificity of the β-mannosidase, BtMan2A. J Biol Chem 282(15):11291–11299
Tailford LE, Ducros VMA, Flint JE, Roberts SM, Morland C, Zechel DL (2009) Understanding how diverse β-mannanases recognize heterogeneous substrates. Biochem 48:7009–7018
Van Zyl WH, Rose SH, Trollope K, Gorgens JF (2010) Fungal β-mannanases: mannan hydrolysis, heterologous production and biotechnological applications. Process Biochem 45:1203–1213
Várnai A, Huikko L, Pere J, Sikka-aho M, Viikari L (2011) Synergistic action of xylanase and mannanase improves the total hydrolysis of softwood. Bioresour Technol 102(19):9096–9104
Wang H, Luo H, Li J, Bai Y, Huang H, Shi P, Fan Y, Yao B (2010) An α-galactosidase from an acidophilic Bispora sp. MEY-1 strain acts synergistically with β-mannanase. Bioresour Technol 101:8376–8382
Wang H, Ma R, Shi P, Huang H, Yang P, Wang Y, Fan Y, Yao B (2015) Insights into the substrate specificity and synergy with mannanase of family 27 α-galactosidases from Neosartorya fischeri P1. Appl Microb Biotechnol 99:1261–1272
Willför S, Sundberg K, Tenkanen M, Holmbom B (2008) Spruce-derived mannans—a potential raw material for hydrocolloids and novel advanced natural materials. Carbohydr Polym 72:197–210
Xiao Z, Zhang X, Gregg DJ, Saddler JN (2004) Effects of sugar inhibition on cellulases and β-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 113–116:1115–1126
Yamabhai M, Sak-Ubol S, Sril W, Dietmar H (2014) Mannan biotechnology: from biofuels to health. Crit Rev Biotechnol 15:1–11
Zahura UA, Mohammad MR, Inoue A, Ojima T (2012) Characterization of a β-mannosidase from a marine gastropod, Aplysia kurodai. Comp Biochem Physiol 162:24–33
Acknowledgments
Financial support from the National Research Foundation (NRF) of South Africa and Rhodes University is gratefully acknowledged. Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore the NRF does not accept any liability in regard thereto.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malgas, S., van Dyk, J.S. & Pletschke, B.I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J Microbiol Biotechnol 31, 1167–1175 (2015). https://doi.org/10.1007/s11274-015-1878-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1878-2