Abstract
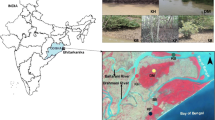
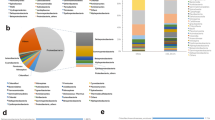
Mangrove microbial communities and their associated activities have profound impact on biogeochemical cycles. Although microbial composition and structure are known to be influenced by biotic and abiotic factors in the mangrove sediments, finding direct correlations between them remains a challenge. In this study we have explored sediment bacterial diversity of the Sundarbans, a world heritage site using a culture-independent molecular approach. Bacterial diversity was analyzed from three different locations with a history of exposure to differential anthropogenic activities. 16S rRNA gene libraries were constructed and partial sequencing of the clones was performed to identify the microbial strains. We identified bacterial strains known to be involved in a variety of biodegradation/biotransformation processes including hydrocarbon degradation, and heavy metal resistance. Canonical Correspondence Analysis of the environmental and exploratory datasets revealed correlations between the ecological indices associated with pollutant levels and bacterial diversity across the sites. Our results indicate that sites with similar exposure of anthropogenic intervention reflect similar patterns of microbial diversity besides spatial commonalities.







Similar content being viewed by others
References
Alain K, Harder J, Widdel F, Zengler K (2012) Anaerobic utilization of toluene by marine alpha- and gammaproteobacteria reducing nitrate. Microbiology 158:2946–2957. doi:10.1099/mic.0.061598-0
Alongi D (2002) Present state and future of the world’s mangrove forests Australian institute marine. Science 29:331–349
Andreote FD et al (2012) The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PloS One 7(e38600):1991. doi:10.1371/journal.pone.0038600PONE-D-12-09292
Aronstein BN, Calvillo, YM, Alexander M (1991) Effect of surfactants at low concentrations on the desorption and biodegradation of sorbed aromatic compounds in soil. Environ Science Technol. 25:1728–1731
Awata T, Tanabe K, Kindaichi T, Ozaki N, Ohashi A (2012) Influence of temperature and salinity on microbial structure of marine anammox bacteria. Water sci technol j Int Assoc Water Pollut Res 66:958–964. doi:10.2166/wst.2012.234
Banerjee K, Senthilkumar B, Purvaja R, Ramesh R (2012) Sedimentation and trace metal distribution in selected locations of Sundarbans mangroves and Hooghly estuary, northeast coast of India. Environ Geochem Health 34:27–42. doi:10.1007/s10653-011-9388-0
Barbier EB et al (2008) Coastal ecosystem-based management with nonlinear ecological functions and values. Science 319:321–323. doi:10.1126/science.1150349
Binelli A et al (2007) Concentration of polybrominated diphenyl ethers (PBDEs) in sediment cores of Sundarban mangrove wetland, northeastern part of Bay of Bengal (India). Mar Pollut Bull 54:1220–1229. doi:10.1016/j.marpolbul.2007.03.021
Black CA (1965) Methods of soil analysis. American Society of Agronomy, Wisconsin
Boonchan S, Britz ML, Stanley GA (1998) Surfactant-enhanced biodegradation of high molecular weight polycyclic aromatic hydrocarbons by stenotrophomonas maltophilia. Biotechnol Bioeng 59:482–494
Braganca I, Placido A, Paiga P, Domingues VF, Delerue-Matos C (2012) QuEChERS: a new sample preparation approach for the determination of ibuprofen and its metabolites in soils. Sci Total Environ 433:281–289. doi:10.1016/j.scitotenv.2012.06.035S0048-9697(12)00861-3
Chaudhuri K, Manna S, Sarma KS, Naskar P, Bhattacharyya S, Bhattacharyya M (2012) Physicochemical and biological factors controlling water column metabolism in Sundarbans estuary, India. Aquat biosyst 8:26. doi:10.1186/2046-9063-8-26
Chouari R, Le Paslier D, Daegelen P, Ginestet P, Weissenbach J, Sghir A (2003) Molecular evidence for novel planctomycete diversity in a municipal wastewater treatment plant. Appl Environ Microbiol 69:7354–7363
Comans RNJ, Van Dijk CPJ (1988) Role of complexation processes in cadmium mobilization during estuarine mixing. Nature 336:151–154
Correia-Sa L, Fernandes VC, Carvalho M, Calhau C, Domingues VF, Delerue-Matos C (2012) Optimization of QuEChERS method for the analysis of organochlorine pesticides in soils with diverse organic matter. J Sep Sci 35:1521–1530. doi:10.1002/jssc.201200087
Davis JW, Gonsior S, Marty G, Ariano J (2005) The transformation of hexabromocyclododecane in aerobic and anaerobic soils and aquatic sediments. Water Res 39:1075–1084. doi:10.1016/j.watres.2004.11.024
Dias ACF et al (2009) Diversity and biotechnological potential of culturable bacteria from Brazilian mangrove sediment. World J Microbiol Biotechnol 27:1305–1311
Dias AC, Andreote FD, Rigonato J, Fiore MF, Melo IS, Araujo WL (2010) The bacterial diversity in a Brazilian non-disturbed mangrove sediment. Antonie Van Leeuwenhoek 98:541–551. doi:10.1007/s10482-010-9471-z
dos Santos HF et al (2011) Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: bacterial proxies for oil pollution. PloS One 6:e16943. doi:10.1371/journal.pone.0016943
Dunsmore B, Youldon J, Thrasher DR, Vance I (2006) Effects of nitrate treatment on a mixed species, oil field microbial biofilm. J Ind Microbiol Biotechnol 33:454–462. doi:10.1007/s10295-006-0095-2
Ehrenreich A, Widdel F (1994) Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl Environ Microbiol 60:4517–4526
Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball MC (2010) Biocomplexity in mangrove ecosystems. Annu Rev Mar Sci 2:395–417
Felsenstein J (1985) Phylogenies and the Comparative Method. Am Nat 125:1–15. doi:10.1086/284325
Feng H, Han X, Zhang W, Yu L (2004) A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Mar Pollut Bull 49:910–915. doi:10.1016/j.marpolbul.2004.06.014
Fernandes VC, Domingues VF, Mateus N, Delerue-Matos C (2013) Multiresidue pesticides analysis in soils using modified QuEChERS with disposable pipette extraction and dispersive solid-phase extraction. J Sep Sci 36:376–382. doi:10.1002/jssc.201200673
Gambrell RP, Wiesepape JB, Patrick WH Jr, Duff MC (1991) The effects of pH, redox, and salinity on metal release from a contaminated sediment. Water Air Soil Pollut 57–58:359–367
Ghosh A, Maity B, Chakrabarti K, Chattopadhyay D (2007) Bacterial diversity of east Calcutta wet land area: possible identification of potential bacterial population for different biotechnological uses. Microb Ecol 54:452–459. doi:10.1007/s00248-007-9244-z
Ghosh A, Dey N, Bera A, Tiwari A, Sathyaniranjan K, Chakrabarti K, Chattopadhyay D (2010) Culture independent molecular analysis of bacterial communities in the mangrove sediment of Sundarban, India. Saline systems 6:1. doi:10.1186/1746-1448-6-1
Giri C, Ochieng E, Tieszen LL, Singh A, Loveland T, Masek J, Deuke N (2011) Status and distribution of mangrove forests of the world using earth observation satellite data. Glob Ecol Biogeogr 20:154–159
Gomes NCM, Borges LR, Paranhos R, Pinto FN, Mendonca-Hagler LC, Smalla K (2008) Exploring the diversity of bacterial communities in sediments of urban mangrove forests. FEMS Microbiol Ecol 66:96–109. doi:10.1111/j.1574-6941.2008.00519.x
Gonzalez-Acosta B, Bashan Y, Hernandez-Saavedra NY, Ascencio F, Cruz-Aguero G (2006) Seasonal seawater temperature as the major determinant for populations of culturable bacteria in the sediments of an intact mangrove in an arid region. FEMS Microbiol Ecol 55:311–321. doi:10.1111/j.1574-6941.2005.00019.x
Gopal B, Chauhan M (2006) Biodiversity and its conservation in the Sundarban mangrove ecosystem. Aquat Sci 69:338–354
Guzzella L, Roscioli C, Vigano L, Saha M, Sarkar SK, Bhattacharya A (2005) Evaluation of the concentration of HCH, DDT, HCB, PCB and PAH in the sediments along the lower stretch of Hugli estuary, West Bengal, northeast India. Environ Int 31:523–534. doi:10.1016/j.envint.2004.10.014
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Han J et al (2005) Characterization of a novel plant growth-promoting bacteria strain Delftia tsuruhatensis HR4 both as a diazotroph and a potential biocontrol agent against various plant pathogens. Syst Appl Microbiol 28:66–76
Handley KM et al (2012) Biostimulation induces syntrophic interactions that impact C, S and N cycling in a sediment microbial community. ISME j. doi:10.1038/ismej.2012.148
Hollister EB, Engledow AS, Hammett AJ, Provin TL, Wilkinson HH, Gentry TJ (2010) Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME j 4:829–838. doi:10.1038/ismej.2010.3
Kathiresan K, Qasim SZ (2005) Biodiversity of mangrove ecosystems. Hindustan Publishing Corporation, New Delhi
Kim JS, Makama M, Petito J, Park NH, Cohan FM, Dungan RS (2012) Diversity of Bacteria and Archaea in hypersaline sediment from Death Valley National Park, California. MicrobiologyOpen 1:135–148. doi:10.1002/mbo3.20
Knap A, Michaels A, Close A, Ducklow H, Dickson A (eds) (1996) Protocols for the joint global ocean flux study (JGOFS) core measurements. JGOFS Report Nr. 19, p 170 (Reprint of the IOC Manuals and Guides No. 29, UNESCO, 1994)
Knudsen M (1901) Hydrographical tables. GEC Gad Cph
Kondo R, Mori Y, Sakami T (2012) Comparison of sulphate-reducing bacterial communities in Japanese fish farm sediments with different levels of organic enrichment. Microbes environ/JSME 27:193–199
Lane DJ (1991) 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics, Willey
Lee JC, Lee GS, Park DJ, Kim CJ (2008) Bacillus alkalitelluris sp. nov., an alkaliphilic bacterium isolated from sandy soil. Int J Syst Evol Microbiol 58:2629–2634. doi:10.1099/ijs.0.65733-0
Lefebvre O, Vasudevan N, Thanasekaran K, Moletta R, Godon JJ (2006) Microbial diversity in hypersaline wastewater: the example of tanneries. Extremophiles life under extrem cond 10:505–513. doi:10.1007/s00792-006-0524-1
Liang J-B, Chen YQ, Lan C-Y, Tam NFY, Zan Q-J, Huang L-N (2007) Recovery of novel bacterial diversity from mangrove sediment. Mar Biol 150:739–747
Long ER, Morgan LG (1991) The potential for biological effects of sediment-sorbed contaminants tested in the national status and trends program. Second edn, National Oceanic and Atmospheric Administration (NOAA), Seattle, Washington
Manna S, Chaudhuri K, Bhattacharyya S, Bhattacharyya M (2010) Dynamics of Sundarban estuarine ecosystem: eutrophication induced threat to mangroves. Saline syst 6:8. doi:10.1186/1746-1448-6-8
Manna M, Chaudhuri K, Sen SK, Naskar P, Bhattacharyya S, Bhattacharyya M (2012) Interplay of physical, chemical and biological components in estuarine ecosystem with special reference to Sundarbans, India. Ecological water quality: water treatment and reuse, InTech
Massolo S, Bignasca A, Sarkar SK, Chatterjee M, Bhattacharya BD, Alam A (2012) Geochemical fractionation of trace elements in sediments of Hugli River (Ganges) and Sundarban wetland (West Bengal, India). Environ Monit Assess 184:7561–7577. doi:10.1007/s10661-012-2519-y
Mesbah NM, Abou-El-Ela SH, Wiegel J (2007) Novel and unexpected prokaryotic diversity in water and sediments of the alkaline, hypersaline lakes of the Wadi An Natrun, Egypt. Microb Ecol 54:598–617. doi:10.1007/s00248-006-9193-y
Moune S, Caumette P, Matheron R, Willison JC (2003) Molecular sequence analysis of prokaryotic diversity in the anoxic sediments underlying cyanobacterial mats of two hypersaline ponds in Mediterranean salterns. FEMS Microbiol Ecol 44:117–130. doi:10.1016/S0168-6496(03)00017-5
Mummey DL, Stahl PD, Buyer JS (2002) Microbial biomarkers as an indicator of ecosystem recovery following surface mine reclamation. Appl Soil Ecol 21:251–259
Nelson DW, Somers LE (1975) Organic carbon. Methods in soil microbiology and biochemistry. Academic, London
Paalman MAA, Van Der Weijden CH, Loch JPG (1994) Sorption of cadmium on suspended matter under estuarine conditions; competition and complexation with major sea-water ions. Water Air Soil Pollut 73:49–60
Quast C et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi:10.1093/nar/gks1219
Rahman MT, Rahman MS, Quraishi SB, Ahmad JU, Choudhury TR, Mottaleb MA (2011) Distribution of heavy metals in water and sediments in Passur River Sundarban mangrove forest, Bangladesh. J Int Environ Appl Sci 6:537–546
Ramond JB, Welz PJ, Cowan DA, Burton SG (2012) Microbial community structure stability, a key parameter in monitoring the development of constructed wetland mesocosms during start-up. Res Microbiol 163:28–35. doi:10.1016/j.resmic.2011.09.003
Reshetnikov AS, Khmelenina VN, Mustakhimov II, Kalyuzhnaya M, Lidstrom M, Trotsenko YA (2011) Diversity and phylogeny of the ectoine biosynthesis genes in aerobic, moderately halophilic methylotrophic bacteria. Extremophiles life under extrem cond 15:653–663. doi:10.1007/s00792-011-0396-x
Romano E, Ausili A, Zharova N, Magno MC, Pavoni B, Gabellini M (2004) Marine sediment contamination of an industrial site at Port of Bagnoli, Gulf of Naples, Southern Italy. Mar Pollut Bull 49:487–495. doi:10.1016/j.marpolbul.2004.03.014
Roy S, Hens D, Biswas D, Biswas D, Kumar R (2002) Survey of petroleum: degrading bacteria in coastal waters of Sunderban. Biosph Reserve World J Microbiol Biotech 18:575–581
Saha M, Sarkar SK, Bhattacharya B (2006) Interspecific variation in heavy metal body concentrations in biota of Sunderban mangrove wetland, northeast India. Environ Int 32:203–207. doi:10.1016/j.envint.2005.08.012
Santos IR, Silva-Filho EV, Schaefer CE, Albuquerque-Filho MR, Campos LS (2005) Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar Pollut Bull 50:185–194. doi:10.1016/j.marpolbul.2004.10.009
Santos HF, Carmo FL, Paes JES, Rosado AS, Peixoto RS (2010) Bioremediation of Mangroves impacted by petroleum. Water Air Soil Pollut 216:329–350
Santschi PH (1984) Particle flux and trace metal residence time in natural water. Limnol Oceanogra 29:1100–1108
Sarkar SK, Bhattacharya B, Debnath S, Bandopadhaya G, Giri S (2002) Heavy metals in biota from Sundarban wetland ecosystem, India: implications to monitoring and environmental assessment. Aquat Ecosyst Health Manage 5:467–472
Sarkar SK, Franciskovic-Bilinski S, Bhattacharya A, Saha M, Bilinski H (2004) Levels of elements in the surficial estuarine sediments of the Hugli River, northeast India and their environmental implications. Environ Int 30:1089–1098. doi:10.1016/j.envint.2004.06.005S0160-4120(04)00114-X
Satpathy KK, Mohanty AK, Prasad MVR, Natesan U, Sarkar SK (2012) Studies on the variations of heavy metals in the marine sediments off Kalpakkam, East Coast of India. Environ Earth Sci 65:89–101
Schloss PD et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi:10.1128/AEM.01541-09
Sorokin DY et al (2001) Thioalkalimicrobium aerophilum gen. nov., sp. nov. and Thioalkalimicrobium sibericum sp. nov., and Thioalkalivibrio versutus gen. nov., sp. nov., Thioalkalivibrio nitratis sp.nov., novel and Thioalkalivibrio denitrificancs sp. nov., novel obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria from soda lakes. Int J Syst Evol Microbiol 51:565–580
Sorokin DY et al (2012) Nitrification expanded: discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME j 6:2245–2256. doi:10.1038/ismej.2012.70
Sousa OV, Macrae A, Menezes FG, Gomes NC, Vieira RH, Mendonca-Hagler LC (2006) The impact of shrimp farming effluent on bacterial communities in mangrove waters, Ceara, Brazil. Mar Pollut Bull 52:1725–1734. doi:10.1016/j.marpolbul.2006.07.006
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Thompson CE, Beys-da-Silva WO, Santi L, Berger M, Vainstein MH, Guima Raes JA, Vasconcelos AT (2013) A potential source for cellulolytic enzyme discovery and environmental aspects revealed through metagenomics of Brazilian mangroves. AMB Express 3:65. doi:10.1186/2191-0855-3-65
Tian Y, Liu HJ, Zheng TL, Kwon KK, Kim SJ, Yan CL (2008) PAHs contamination and bacterial communities in mangrove surface sediments of the Jiulong River Estuary, China. Mar Pollut Bull 57:707–715. doi:10.1016/j.marpolbul.2008.03.011
Wang P, Li X, Xiang M, Zhai Q (2007) Characterization of efficient aerobic denitrifiers isolated from two different sequencing batch reactors by 16S-rRNA analysis. J Biosci Bioeng 103:563–567. doi:10.1263/jbb.103.563
Yoshie S, Makino H, Hirosawa H, Shirotani K, Tsuneda S, Hirata A (2006) Molecular analysis of halophilic bacterial community for high-rate denitrification of saline industrial wastewater. Appl Microbiol Biotechnol 72:182–189. doi:10.1007/s00253-005-0235-z
Zocca C, Di Gregorio S, Visentini F, Vallini G (2004) Biodiversity amongst cultivable polycyclic aromatic hydrocarbon-transforming bacteria isolated from an abandoned industrial site. FEMS Microbiol Lett 238:375–382. doi:10.1016/j.femsle.2004.07.055
Acknowledgments
We acknowledge World Bank, for all the necessary support for the execution of the ICZM project. We would like to acknowledge the continuous encouragement and enthusiasm expressed by Mr. Tapas Paul, Task Team Leader, ICZM project and Dr. Herbert K. Acquay, Sector Manager, Environment Water Resources and Climate Change Sustainable Development, South Asia Region, The World Bank in our venture to explore the world heritage site, Sundarbans. We express our sincere gratitude to State Project Management Unit, ICZMP West Bengal for their continuous support and interest in our research work. We would like to acknowledge the instrument facility provided by UGC-CAS, DST–FIST in Department of Biochemistry and DNA-sequencing facility developed from the NCMP grant to University of Calcutta, India. We would like to thank Ms. Kakoli Mukherjee, IESWM, Kolkata for her help in constructing a working map of the study area of Sundarbans. It would not be possible to carry out this work without the help and support of the local people of the Sundarbans. We express our inability to acknowledge them individually. A.G. was supported by Ramanujan Fellowship from Department of Science and Technology, India (SR/S2/RJN-106/2012).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Arpita Chakraborty, Amit Bera and Arghya Mukherjee have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chakraborty, A., Bera, A., Mukherjee, A. et al. Changing bacterial profile of Sundarbans, the world heritage mangrove: Impact of anthropogenic interventions. World J Microbiol Biotechnol 31, 593–610 (2015). https://doi.org/10.1007/s11274-015-1814-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1814-5