Abstract
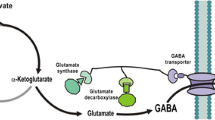
γ-aminobutyric acid (GABA) is an important bioactive regulator, and its biosynthesis is primarily through the α-decarboxylation of glutamate by glutamate decarboxylase (GAD). The procedures to obtain GABA by bioconvertion with high activity recombinant Escherichia coli GAD have been seldom understood. In this study, Escherichia coli GAD (gadA) was highly expressed (about 70–75% of total protein) as soluble protein in Escherichia coli BL21(DE3) containing pET28a-gadA, which was induced by 0.4 mM IPTG in LB medium, and maximal GABA-forming activity of the recombinant GAD was 40 U/mL at a concentration (0.15 mM) of pyridoxal phosphate (PLP) and a concentration (0.6 mM) of Ca2+ at optimal pH of 3.8. The optimal concentration (7.5 mM) of Mn2+ can also improve the activity of recombinant enzyme, but the co-effect of Ca2+ and Mn2+ exhibited antagonism effect when added simultaneously. LB and 0.1% (w/v) lactose were selected as culture medium and inducer, respectively. The relative activity was markedly higher activated by Ca2+ (174%), Mn2+ (164%) than that by other seven bivalent cations. Finally, the yield of GABA was high of 94 g/L detected by paper chromatography or HPLC in 1 L reaction system with 30 mL crude GAD (12 U/mL). By entrapping Escherichia coli glutamate decarboxylase into sodium alginate and carrageenan gel beads, the activity of immobilized GAD (IGAD) remained 85% during the initial five batches and the activity still remained 50% at the tenth batch, these results indicated that the recombinant Escherichia coli GAD was feasible for the future industrial production of GABA.







Similar content being viewed by others
References
Adeghate E, Ponery AS (2002) GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34:1–6. doi:10.1054/tice.2002.0217
Bai QY, Chai MQ, Gu ZX, Cao XH, Li Y, Liu KL (2009) Effects of components in culture medium on glutamate decarboxylase activity and γ-aminobutyric acid accumulation in foxtail millet (Setaria italica L.) during germination. Food Chem 116:152–157. doi:10.1007/s00217-008-0920-0
Bartlett PN, Cooper JM (1993) A review of the immobilization of enzymes in electropolymerized films. J Electroanal Chem 362:1–12. doi:10.1016/0022-0728(93)80001-X
Battaglioli G, Liu H, Martin DL (2003) Kinetic differences between the isoforms of glutamate decarboxylase: implications for the regulation of GABA synthesis. J Neurochem 86:879–887. doi:10.1046/j.1471-4159.2003.01910.x
Baum G, Chen Y, Arazi T, Takatsuji H, Fromm H (1993) A plant glutamate decarboxylase containing a calmodulin binding domain. Cloning, sequence, and functional analysis. J Biol Chem 268:19610–19617
Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H (1996) Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J 15:2988–2996
Capitani G, De Biase D, Aurizi C, Gut H, Bossa F, Grütter MG (2003) Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J 22:4027–4037. doi:10.1093/emboj/cdj403
Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW (1999) Control of acid resistance in Escherichia coli. J Bacteriol 181:3525–3535
Chen XX, Li D, Lv JX, Fang F (1997) Determination of γ-aminobutyric acid and glutamic acid in human cerebrospinal fluid by high performance liquid chromatography (in Chinese). Chin J Chromatogr 15:237–239. doi:1000-8713.0.1997-03-016
Choi SI, Lee JW, Park SM, Lee MY, Ji GE, Park MS, Heo TR (2006) Improvement of gamma-aminobutyric acid (GABA) production using cell entrapment of Lactobacillus brevis GABA 057. J Microbiol Biotechnol 16:562–568
Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R (2002) On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 298:1418–1421. doi:10.1126/science.1076510
Coleman ST, Fang TK, Rovinsky SA, Turano FJ, Moye-Rowley WS (2001) Expression of a glutamate decarboxylase homologue is required for normal oxidative stress tolerance in Saccharomyces cerevisiae. J Biol Chem 276:244–250. doi:10.1074/jbc.M007103200
Crivici A, Ikura M (1995) Molecular and structural basis of target recognition by calmodulin. Annu Rev Biophys Biomol Struct 24:85–116. doi:10.1146/annurev.bb.24.060195.000505
David GK, Michael EM (2003) Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev 27:263–290. doi:10.1016/S0168-6445(03)00052-4
De Biase D, Tramonti A, Bossa F, Visca P (1999) The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol Microbiol 32(6):1198–1211. doi:10.1046/j.1365-2958.1999.01430.x
Fitsanakis VA, Aschner M (2005) The importance of glutamate, glycine, and γ-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharmacol 204(3):343–354. doi:10.1016/j.taap.2004.11.013
Foester CW, Foester HF (1973) Glutamic acid decarboxylase in spores of Bacillus megaterium and its possible involvement in spore germination. J Bacteriology 114:1090–1098
Hagiwara H, Seki T, Ariga T (2004) The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 68:444–447. doi:10.1271/bbb.68.444
Huang J, Mei LH, Wu H, Lin DQ (2006) Biosynthesis of γ-aminobutyric acid (GABA) using immobilized whole cells of Lactobacillus brevis. World J Microbiol Biotechnol 23:865–871. doi:10.1007/s11274-006-9311-5
Jakobs C, Jaeken J, Gibson KM (1993) Inherited disorders of GABA metabolism. J Inherit Metab Dis 16:704–715. doi:10.1007/BF00711902
Komatsuzaki N, Shima J, Kawamoto S, Momose H, Kimura T (2005) Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol 22:497–504. doi:10.1016/j.fm.2005.01.002
Krnjevic K (1974) Chemical nature of synaptic transmission in vertebrates. Physiol Rev 54:419–540
Ling DR, Wu GQ, Wang C, Wang F, Song GQ (2000) The preparation and characterization of an immobilized L-glutamic decarboxylase and its application for determination of L-glutamic acid. Enzyme Microb Technol 27(7):516–521. doi:10.1016/S0141-0229(00)00242-8
Liu Q, Yao HY, Zhang H (2005) Studies on some properties of glutamate decarboxylase from lactic acid bacterium. Food Science 26:100–104. doi:1002-6630.0.2005-04-021 (in Chinese)
Marko-Varga G, Dominguez E (1991) Enzymes as analytical tools. Trends Anal Chem 10:290–297. doi:10.1016/0165-9936(91)85007-E
Matsumoto T, Yamaura I, Funatsu M (1986) Purification and properties of glutamate decarboxylase from Squash. Agric Biol Chem 50(6):1413–1417
Nomura M, Kimoto H, Someya Y, Furukawa S, Suzuki I (1998) Production of gamma-aminobutyric acid by cheese starters during cheese ripening. J Dairy Sci 81:1486–1491
Noriyoshi T, Katanori N (2002) Method for producing γ-aminobutyric acid. Jpn Pat, 332134
O’Hara T, Rajagopalan R, Heller A (1994) Wired enzyme electrodes for amperometric determination of glucose or lactate in the presence of interfering substances. Anal Chem 66:2451–2457. doi:10.1021/ac00087a008
Plokhov AY, Gusyatiner MM, Yampolskaya TA, Kaluzhsk VE, Sukhareva BS, Schulga AA (2000) Preparation of gamma-aminobutyric acid using E. coli cells with high activity of glutamate decarboxylase. Appl Biochem Biotechnol 88:257–265. doi:10.1385/ABAB:88:1-3:257
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. In: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York, pp 880–898
Sanders JW, Leenhouts K, Burghoorn J, Brands JR, Venema G, Kok J (1998) A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol 27:299–310. doi:10.1046/j.1365-2958.1998.00676.x
Satyanarayan V, Nair PM (1985) Purification and characterization of glutamate decarboxylase from Solanum tuberosum. Eur J Biochem 150:53–60. doi:10.1111/j.1432-1033.1985.tb08987.x
Satyanarayan V, Nair PM (1990) Metabolism, enzymology and possible roles of γ-aminobutyrate in higher plants. Phytochem 29:367–375. doi:10.1016/0031-9422(90)85081-P
Sawai Y, Yamaguchi Y, Miyama D, Yoshitomi H (2001) Cycling treatment of anaerobic and aerobic incubation increases the content of γ-aminobutyric acid in tea shoots. Amino Acids 20:331–334. doi:10.1007/s007260170049
Simonian AL, Badalian IE, Berezov TT, Smirnova IP, Khaduev SH (1994) Flow-injection amperometric biosensor based on immobilized L-lysine-a-oxidase for L-lysine determination. Anal Lett 27:2849–2860. doi:10.1080/00032719408000296
Snedden WA, Koutsia N, Baum G, Fromm H (1996) Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes thecalmodulin binding domain. J Biologi Chem 271:4148–4153. doi:10.1074/jbc.271.8.4148
Sukhareva BS, Tikhonenko AS, Dariĭ EL (1994) Study of the quaternary structure of glutamate carboxylase from Escherichia coli. Mol Biol 28:1407–1411
Tong JC, Mackay IR, Chin J, Law RHP, Fayad K, Rowley MJ (2002) Enzymatic characterization of a recombinant isoform hybrid of glutamic acid decarboxylase (rGAD67/65) expressed in yeast. J Biotech 97:183–190. doi:10.1016/S0168-1656(02)00060-3
Ueno H (2000) Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal 10:67–79. doi:10.1016/S1381-1177(00)00114-4
Warnecke T, Gill YT (2005) Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Fact 4:1–8. doi:10.1186/1475-2859-4-25
Weinstein H, Mehler EL (1994) Ca2+-binding and structural dynamics in the functions of calmodulin. Annu Rev Physiol 56:213–236. doi:10.1146/annurev.ph.56.030194.001241
Wen ML, Zhao YB, Wang CY (1997) Research advances in electro-analytical chemistry for life science. Yunnan Chem Technol 2:3–8. doi:1004-275X.0.1997-02-001 (in Chinese)
Woolfolk CA, Shapiro B, Stadtman ER (1966) Regulation of glutamine synthetase I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys 116:177–192. doi:10.1016/0003-9861(66)90026-9
Wu LG, Liu ME, Zhu CL (1995) Research, and development of biosensors. Prog Chem 7:287–310. doi:113383O6.0.1995-04-002 (in Chinese)
Xie ZD, Bao FW, Li MQ, He BL (1995) Immobilization of porcine pancreatic lipase on macro-porous polymethyl acrylate resin. Ion Exch Adsorp 11:24–29. doi:10015493.0.1995-01-005 (in Chinese)
Yamada H, O’Leary MH (1977) A solvent isotope effect probe for enzyme-mediated proton transfers. J Am Chem Soc 99:1660–1661. doi:10.1021/ja00447a071
Yokoyama S, Hiramatsu J, Hayakawa K (2002) Production of gamma-aminobutyric acid from alcohol distillery lees by Lactobacillus brevis IFO–12005. J Biosci Bioeng 93:95–97. doi:10.1016/S1389-1723(02)80061-5
Zhang H, Yao HY, Chen F, Wang X (2007) Purification and characterization of glutamate decarboxylase from rice germ. Food Chem 101:1670–1676. doi:10.1016/j.foodchem.2006.04.027
Acknowledgments
This work was supported by the special funding from NJNU for talent faculty. We thank zhili Liu, associate professor, for performing the HPLC analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Q., Xin, Y., Zhang, F. et al. Enhanced γ-aminobutyric acid-forming activity of recombinant glutamate decarboxylase (gadA) from Escherichia coli . World J Microbiol Biotechnol 27, 693–700 (2011). https://doi.org/10.1007/s11274-010-0508-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0508-2