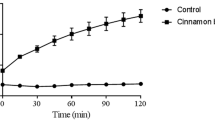
Abstract
Plants are known to produce a plethora of secondary metabolites which are recognized as a useful source of new drugs or drug leads. Extracts and fractions of Schinus terebinthifolius Raddi (Anacardiaceae), Piper regnellii C.D.C. (Piperaceae), Rumex acetosa L. (Polygonaceae), and Punica granatum L. (Punicaceae) were assessed for their antifungal activity against eight clinical isolates of C. albicans. They were also evaluated for their effect on the adhesion of these C. albicans isolates to buccal epithelial cells (BECs). The ethyl acetate fraction from the leaves of S. terebinthifolius showed promising activity, inhibiting the growth of three C. albicans isolates at 7.8 μg ml−1 and significantly inhibiting their adhesion to BEC at 15 μg ml−1 . In addition, this fraction did not show cytotoxic activity against murine macrophages. The results show the potential of the plant extracts studied as a source of new antifungal compounds. Further studies are necessary for isolation and characterization of the active compounds of these plants.

Similar content being viewed by others
References
Alviano WS, Mendonça-Filho RR, Alviano DS et al (2005) Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol Immun 20:101–105. doi:10.1111/j.1399-302X.2004.00201.x
Arango ACM, Sánchez JGB, Galviz LAB (2004) Productos Naturales com actividad antimicótica. Rev Esp Quimioter 17:325–331
Bendel CM (2003) Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Semin Perinatol 27:357–364. doi:10.1016/S0146-0005(03)00059-4
Dorocka-Bobkowska B, Konopka K, Düzgünes N (2003) Influence of antifungal polyenes on the adhesion of Candida albicans and Candida glabrata to human epithelial cells in vitro. Arch Oral Biol 48:805–814. doi:10.1016/S0003-9969(03)00174-2
Drago L, Mombelli B, De Vecchi E et al (2000) Candida albicans cellular internalization: a new pathogenic factor? Int J Antimicro Ag 16:545–547. doi:10.1016/S0924-8579(00)00296-X
Ellepola ANB, Samaranayake LP (1998a) Adhesion of oral C. albicans to human buccal epithelial cells following limited exposure to antifungal agents. J Oral Pathol Med 27:325–332
Ellepola ANB, Samaranayake LP (1998b) Adhesion of oral Candida albicans isolates to denture acrylic following limited exposure to antifungal agents. Arch Oral Biol 43:999–1007. doi:10.1016/S0003-9969(98)00075-2
Espinel-Ingroff A (1998) Comparison of in vitro activities of the new triazole SCH56592 and echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol 36:2950–2956
Filoche SK, Soma K, Sissons CH (2005) Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immun 20:221–225. doi:10.1111/j.1399-302X.2005.00216.x
Guerra MJM, Barreiro ML, Rodríguez ZM, Rubalcaba Y (2005) Actividad antimicrobiana de um extracto fluido al 80 % de Schinus terebinthifolius Raddi (Copal). Rev Cubana Med Trop 5:23–25
Holetz FB, Pessini GL, Sanches NG et al (2002) Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz 97:1027–1031. doi:10.1590/S0074-02762002000700017
Hussein SAM, Barakat HH, Merfort I, Nawwar MAM (1997) Tannins from the leaves of Punica granatum. Phytochemistry 45:819–823
Johann S, Pizzolatti MG, Resende MA (2005) Antifungal activity of medicinal plant extract. In: Proceedings of the 23th annual meeting of the Brazilian microbiology, Santos, BR. Abstract, 01/740-1. Brazilian Society for Microbiology, São Paulo, SP, BR
Johann S, Pizzolatti MG, Donnici CL, Resende MA (2007a) Antifungal properties of plants used in Brazilian traditional medicine against clinically relevant fungal pathogens. Braz J Microbiol 38:632–637. doi:10.1590/S1517-83822007000400010
Johann S, Soldi C, Lyon JP et al (2007b) Antifungal activity of the amyrin derivatives and in vitro inhibition of Candida albicans adhesion to human epithelial cells. Lett Appl Microbiol 45:148–153. doi:10.1111/j.1472-765X.2007.02162.x
Kimura LH, Pearsall NN (1978) Adherence of Candida albicans to human buccal epthelial cells. Infect Immun 21:64–68
Lafon H, Al-Karaawi ZMC, Collough M et al (2005) Composition of in vitro denture plaques biofilms and susceptibility to antifungals. FEMS Microbiol Lett 242:341–345
Lee NJ, Choi JH, Koo BS et al (2005) Antimutagenicity and cytotoxicity of the constituents from the aerial parts of Rumex acetosa. Biol Pharm Bull 28:2158–2161. doi:10.1248/bpb.28.2158
Lima EO, Freira KRL, Farias NMP (2002) Avaliação da atividade antimicrobiana do extrato aquoso de Punica granatum L. (Punicaceae). Infarma 14:46–49
Lyon JP, Resende MA (2006) Correlation between adhesion, enzyme production, and susceptibility to fluconazole in Candida albicans obtained from denture wearers. Oral Surg Oral Med O 102:632–638
Martinez MJ, Betancourt J, Alonso-Gonzfilez N, Jauregui A (1996) Screening of some Cuban medicinal plants activity for antimicrobial. J Ethnopharmacol 52:171–174. doi:10.1016/0378-8741(96)01405-5
National Committee for Clinical Laboratory Standards (2002) Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. NCCLS, Villanova, PA, USA
Nikawa H, Equsa H, Makihira S et al (2006) An in vitro evaluation of the adhesion of Candida species to oral and lung tissue cells. Mycoses 49:14–17. doi:10.1111/j.1439-0507.2005.01176.x
Olsen I (1990) Oral adhesion of yeast. Acta Odontol Scand 48:39–53
Pessini GL, Dias BPF, Nakamura CV, Cortez DAG (2003) Antibacterial activity of extracts and neolignans from Piper regnellii (Miq.) C. DC. var. pallescens (C. DC.) Yunck. Mem Inst Oswaldo Cruz 98:1115–1120. doi:10.1590/S0074-02762003000800025
Pessini GL, Dias BP, Nakamura CV, Cortez DAG (2005) Antifungal activity of the extracts and neolignans from Piper regnellii (Miq.) C.DC. var. pallescens (C.DC.) Yunck. J Braz Chem Soc 16:1130–1133. doi:10.1590/S0103-50532005000700007
Pfaller MA, Gerarden T, You M, Wenzel RP (1998) Influence of in vitro susceptibility testing conditions on the anti-candidal activity of LY121019. Diagn Microbiol Infect Dis 11:1–9. doi:10.1016/0732-8893(88)90067-3
Polaquini SRB, Svidzinski TIE, Kemmelmeier C (2006) Effect of aqueous extract from Neem (Azadirachta indica A. Juss) on hydrophobicity, biofilm formation and adhesion in composite resin by Candida albicans. Arch Oral Biol 51:482–490. doi:10.1016/j.archoralbio.2005.11.007
Paulnock DM (2000) Macrophages: A practical approach. Oxford University Press, USA
Portillo A, Vila R, Freixa B et al (2005) Antifungal sesquiterpene from the root of Vernonanthura tweedieana. J Ethnopharmacol 97:49–52. doi:10.1016/j.jep.2004.09.052
Prashanth D, Asha MK, Amit A (2001) Antibacterial activity of Punica granatum. Fitoterapia 72:171–173. doi:10.1016/S0367-326X(00)00270-7
Richards RME, Liu X (1994) Antibacterial activity of compounds from Rubus pinfaensis. Planta Med 420–424
Schomourlo G, Mendonça-Filho RR, Alviano CS, Costa SS (2006) Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J Ethnopharmacol 96:563–568. doi:10.1016/j.jep.2004.10.007
Silva JP, Siqueira AM (2000) Accíon antibacteriana de extratos hidroalcohólicos de Rubus urticaefolius. Rev Cubana Plant Med 5:26–29
Soto K, Garza KM, Murr LE (2007) Cytotoxy effects of aggregated nanomaterials. Acta Biomater 3:351–358. doi:10.1016/j.actbio.2006.11.004
Taweechaisupapong S, Choopan T, Singhara S et al (2005) In vitro inhibitory effect of Streblus asper leaf-extract on adhesion of Candida albicans to human buccal epithelial cells. J Ethnopharmacol 96:221–226. doi:10.1016/j.jep.2004.09.010
Toumy SAA, Rauwald HW (2002) Two ellagitannins from Punica granatum heartwood. Phytochemistry 61:971–974. doi:10.1016/S0031-9422(02)00435-1
Vasconcelos LCS, Sampaio MCC, Sampaio FCS, Higino JS (2003) Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Mycoses 46:192–196. doi:10.1046/j.1439-0507.2003.00884.x
Voravuthikunchai S, Lortheeranuwat A, Jeeju W, Sririrak T, Phongpaichit S, Supawita T (2004) Effective medicinal plants against enterohaemorrhagica Escherichia coli O157:H7. J Ethnopharmacol 94:49. doi:10.1016/j.jep.2004.03.036
Acknowledgements
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) from Brazil for financial support. The authors also thank EPAGRI from Itajaí, SC, Brazil, for providing and identifying some of the plant species and also thanks to Prof. Dr. Daniel Barcellos Falkenberg of the Department of Botany of UFSC who identified the species.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johann, S., Silva, D.L., Martins, C.V.B. et al. Inhibitory effect of extracts from Brazilian medicinal plants on the adhesion of Candida albicans to buccal epithelial cells. World J Microbiol Biotechnol 24, 2459–2464 (2008). https://doi.org/10.1007/s11274-008-9768-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9768-5