Abstract
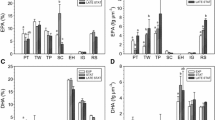
The marine microalga Pavlova viridis (Prymnesiophyceae) is widely used in marine aquaculture industries of China for feeding bivalves and has been proposed as an alternative source of eicosapentaenoic acid (EPA). To investigate variation of its lipid and fatty acid compositions during laboratory and outdoor cultivation, a 60-1 photobioreactor was established in Nanjing, China. Outdoor cultivation, paralleled with laboratory cultures in mid-October, was performed from autumn through midwinter. The results showed that the total lipid and EPA contents of outdoor cultures were both lower than those of indoor cultures. When the outdoor temperature and illumination decreased, total lipid experienced no significant change. Although the level of saturated fatty acids decreased, polyunsaturated fatty acids, especially EPA, increased.





Similar content being viewed by others
References
Belarbi EH, Molina E, Chisti Y (2000) A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzyme Microb Tech 26:516–529
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1995) Microalgae as sources of pharmaceuticals and other biologically active compounds. J Appl Phycol 7:3–15
Bousfield IJ, Smith GL, Dando TR, Hobbs G (1983) Numerical analysis of total fatty acid profiles in the identification of Coryneform, Nocardioform and some other bacteria. J Gen Microbiol 129:375–394
Cohen Z, Cohen S (1991) Preparation of eicosapentaenoic acid (EPA) concentrate from Porphyridium cruentum. Am Oil Chem Soc 68:16–19
Cohen Z (1994) Production potential of eicosapentaenoic acid by Monodus subterraneus. J Am Oil Chem Soc 71:941–945
Cohen Z, Norman HA, Heimer YM (1995) Microalgae as a source of ω-3 fatty acids. In: Simopoulos AP (ed) Plants in human nutrition, vol 77. Karger Basel, pp 1–32
Dong LH, You JT, Lin QQ, Hu R. Han BP (2004) Comparison of fatty acids composition in marine and freshwater microalgae. J Trop Subtrop Bot 12:226–232
Guillard RRL, Ryther JH (1962) Studies on marine planktonic diatoms I. Cyclotella nana Hustedt and Detonula comfervacea (Cleve). Gran Can J Microbiol 8:229–239
Hua XM, Zhou HQ, Ding ZP (1999) Effect of temperature and illumination on the microalgae’s growth, total lipid and fatty acid composition. J Shanghai Fish Univ 8:309–315
James CM, Al-Hinty S, Salman AE (1989) Growth and n-3 fatty acid and amino acid composition of microalgae under different temperature regimes. Aquaculture 77:337–351
Jiang XM, Wang GC, Zhong MJ (1997) A testing Study on ecological conditions of cultivation of Pavlova viridis. J Zhejiang College Fis 16:175–182
Kogteva GS, Beruglov VV (1998) Unsaturated fatty acids as endogenous bioregulators. Biochemistry-Moscow 63:4–12
López-Elías JA, Voltolina D, Chavira-Ortega CO, Rodríguez-Rodríguez BB, Sánz-Gaxiola LM, Cordero-Esquivel B, Nieves M (2003) Mass production of microalgae in six commercial shrimp hatcheries of the Mexican northwest. Aquacult Eng 29:155–164
Mortensen SH, Borsheim KY, Rainuzzo JK, Knutsen G (1988) Fatty acid and elemental composition of the marine diatom Chaetoceros gracilis Schutt. Effects of silicate deprivation, temperature and light intensity. J Exp Mar Biol Ecol 122:173–185
Pande SV, Khan RP, Venkitasubramanian TA (1963) Microdetermination of lipids and serum total fatty acid. Anal Biochem 6:415–423
Renaud SM, Zhou HC, Parry DL, Loung-Van T, Woo KC (1995) Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp., (clone T.ISO). J Appl Phycol 7:595–602
Richmond A (1986) Cell response to environmental factors. In: Richmond A (ed) CRC handbook of microalgae mass culture. CRC Press, Boca Raton, pp 69–106
Seto A, Kumasaka K, Hosaka M, Kojima E, Kashiwakura M, Kato T (1992) Production of eicosapentaenoic acid by marine microalgae and its commercial utilization for aquaculture. In: Kyle DJ, Ratledge C (eds) Industrial application of single cell oils. American Oil Chemists’ Society, Champaign, IL, pp 219–234
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Sukenik A, Yamaguchi Y, Livne A (1993b) Alterations in lipid molecular species of the marine eustigmatophyte Nannochloropsis sp. J Phycol 29:620–626
Sukenik A, Zmora O, Carmeli Y (1993a) Biochemical quality of marine unicellular algae with special emphasis on lipid composition. II. Nannochloropsis sp. Aquaculture 117:313–326
Teshima S, Yamasaki S, Kanazawa A, Hirata H (1983) Effects of water temperature and salinity on eicosapenatenoic acid level of marine Chlorella. Bull Jpn Soc Sci Fish 49:805
Thompson PA (1992b) Effect of variation in temperature: II. On the fatty acid composition of eight species of marine-phytoplankton. J Phycol 28:488–497
Tredici MR, Materassi R (1992) From open ponds to vertical alveolar panels: the Italian experience in the development of reactors for the mass cultivation of phototrophic microorganisms. Appl Phycol 4:221–231
Wen Z, Chen F (2000) Production potential of eicosapentaenoic acid by the diatom Nitzchia laveis. Biotechnol Lett 22:27–33
Yang QY, Shi QQ, Chen BL, Wu SG (2002) Study on advancing the growth rate of Pavlova viridis Tseng with plant hormone. J Fujian Teach Univ (Nat Sci) 16: 80–83
Zhao SF, Sun HQ (2004) Studies on the ecological factors to the growth of Pavlova viridis. Fish Sci 23:9–11
Zhao SF, Sun HQ (2005) Effects of nitrogen, phosphorus and three heavy metals on the growth of Pavlova viridis. J Zhanjiang Ocean Univ 25:60–63
Zittelli GC, Lavista F, Bastianini A, Rodolfi L, Vincenzini M, Tredici MR (1999) Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J Biotecnol 70:299–312
Acknowledgements
This work was supported by grants from the China Postdoctoral Foundation (No. 2005037121) and the Agricultural Three Item Projects of Jiangsu Province (SX (2005) 098). We would like to thank Prof. Y.X. Ou for his kind advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, C., Li, M., Li, J. et al. Variation of lipid and fatty acid compositions of the marine microalga Pavlova viridis (Prymnesiophyceae) under laboratory and outdoor culture conditions. World J Microbiol Biotechnol 24, 1209–1214 (2008). https://doi.org/10.1007/s11274-007-9595-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9595-0