Abstract
This article studied the sorption behavior of Cs(I) ions from aqueous solutions onto molybdenum vanadate@bentonite (MoV@bentonite) composite. MoV@bentonite has been fabricated using the precipitation method and was characterized by different analytical tools including, FT-IR, XRD, and SEM attached with an EDX unit. The sorption studies applied on Cs(I) ions include the effect of contact time, pH, initial metal concentrations, ionic strength, desorption, and recycling. The experimental results revealed that in the adsorption process carried out after equilibrium time (300 min), saturation capacity has a value of 26.72 mg·g−1 and the sorption of Cs(I) ions is dependent on pH values and ionic strength. Sorption kinetic better fit with the pseudo-second-order model; sorption isotherms apply to Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) isotherm models. Data of thermodynamic parameters indicate that sorption is spontaneous and endothermic. Recycling experiments show that MoV@bentonite could be used for 7 cycles and the best eluant for the recovery of Cs(I) ions is 0.1 M HCl (76.9%). All the obtained data clarify that MoV@bentonite is considered a promising sorbent for the sorption of Cs(I) ions from aqueous solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the fission reactions, several corrosive effluents containing radionuclides either short or long-life elements are produced. Removal of these radionuclides from radioactive waste has recently gained great attention as they became more profound with increasing radiation activities. Cesium is one of these pollutants, and it is found in radioactive waste from industry and medicine, as well as research and nuclear facilities, necessitating its separation from aqueous solution. In addition to some toxic organic compounds that are suspected to be carcinogens or allergens, these compounds can cause fatal damage (Abdel Rahman et al. 2019; Lin et al. 2020). Cesium is usually present in water in the cationic form with high mobility and solubility rates; thus, it can be mobile and bioaccumulate in animals and plants which affects the food chain (Noli et al. 2021). Although human health is hardly affected by stable cesium isotopes, the ingestion of Cs-134 leads to a serious disorder of human health and even death; the main reason is due to its chemical similarity to sodium (Na) which leads to its precipitation in the soft tissues in each part of the body which leads to internal hazards (Ghaly et al. 2018, 2022; Noli et al. 2021). Many physicochemical techniques have been developed for the decontamination of Cs-134 or retardation of its migration in the environment, such as precipitation/coprecipitation, reverse osmosis, ultrafiltration, adsorption, solvent extraction, and ion exchange either using synthetic or natural sorbents (Ahn et al. 2020; Lin et al. 2020; Noli et al. 2021; Şenol and Şimşek 2022). The sorption approach is widely used in the disposal of radionuclide-bearing wastewater due to its multiple superiorities of easy operation, low cost, high availability, and favorable removal performance (Veliscek-Carolan et al. 2013; Sankararamakrishnan et al. 2014; Karthik and Meenakshi 2015).
Scholars are particularly interested in the sorption of radionuclides onto natural clay minerals due to their abundant outputs, low cost, good thermal and mechanical stability, nontoxicity, eco-friendliness, and ion exchange capability (Zhang et al. 2019; Huang et al. 2020). Natural rocks and minerals can be used as adsorbent materials, such as bentonite, zeolite, and dolomite which are used by many researchers as adsorbents in the removal of Cs(I) ions from aqueous solutions due to their low cost (Belousov et al. 2019; Ibrahim et al. 2021; Şenol and Şimşek 2022). Bentonite is a natural aluminum phyllosilicate clay composed mainly of montmorillonite which belongs to the smectite group (Wan Ngah et al. 2011; Iwai and Hashimoto 2017; Wahab et al. 2019). The most characteristic property of the smectite group is their tendency to swell (i.e., expand their crystal structure) when reacted with aqueous and polar molecules by entering water or polar molecules in the interlayer structure. All the smectite group minerals are negatively charged on their external surface thus which describes their desirability to adsorb positively charged metal ions (Andrunik and Bajda 2019). The crystal structure of bentonite is composed of an octahedral alumina sheet (O) sandwiched between two contradicting tetrahedral (T) silicate sheets, 2:1 (T-O-T). The length of these sheets is several microns while the thickness is a few nanometers, and the gap in between is filled with exchangeable cations (Brigatti et al. 2013). Bentonite is widely used as a sorbent material for the decontamination of metal ions from wastewater because of its characteristic chemical and physical properties, (i.e., large specific surface area, high cation exchange capacity, and high desirability toward organic and inorganic ions) (Donat et al. 2005; Yang et al. 2014; Wahab et al. 2019; Şenol and Şimşek 2022).
There are many studies on the adsorption of Cs(I) ions in many inorganic sorbents such as Ti–Ca–Mg phosphates (Ivanets et al. 2020), sediment (Fuller et al. 2014), bentonites (Izosimova et al. 2022), and monetite (El-Din et al. 2018). But there were no studies carried out in the sorption of Cs(I) onto a novel MoV@bentonite composite that had not been prepared before. The use of modified bentonite was reported by (Olu-Owolabi and Unuabonah 2011; Dinh et al. 2022; Yang et al. 2022; Zhou et al. 2022). However, there are no reports on the adsorption capacity and the mechanism of MoV@bentonite as a sorbent material.
The novelty of this study is the decontamination of cesium-134 using MoV@bentonite which is a low-cost sorbent material with high reliability and performance. To overcome the negative feature of bentonite and to increase its interest in terms of adsorption, inorganic sorbent-bentonite composites are synthesized. The object of this study is to include the evaluation of a novel MoV@bentonite for cesium-134 decontamination from aqueous solutions. Different analytical tools were used to characterize the MoV@bentonite composite.
Experiment
Materials
Cesium chloride (CsCl, 99.99%), sodium vanadate (NaVO3, 98%), and sodium molybdate (Na2MoO4·2H2O, 99%) were supplied from Alpha Chemika, India; sodium tripolyphosphate (Na5P3O10, 95%) was supplied from Goway, China; nitric acid (HNO3, 65%) and hydrochloric acid (HCl, 35%) were supplied from Merck, Germany; and sodium hydroxide (NaOH, 99%) and ammonium hydroxide (NH4OH, 99%) were supplied from El-Nasr Co, Egypt. All chemicals and reagents used in this work were used without further purification. A stock solution of 1000 mg L−1 Cs(I) ions was prepared by dissolving the required amount CsCl in a definite volume of double distilled water.
Radiotracer preparation
The second Egyptian training research reactor, ETRR-2 at Inshas, Egypt, was used to obtain 134Cs radioactive tracers. Specific grams of cesium chloride was wrapped in a thin thickness high-purity aluminum foil; then, it was held in a thick aluminum irradiation capsule after that the capsule was subjected to a pile of neutrons in the reactor. A definite quantity of the irradiated cesium chloride was dissolved in double distilled water. Equilibrium measurements were performed using the resulting isotopes as tracers. A single-channel analyzer supplied with a well-type NaI(Tl) detector was used to detect the γ-radiation radioactivity of the obtained isotopes.
Preparation
For the preparation of bentonite, molybdenum vanadate) MoV(, and MoV@bentonite composites, a simple and ambient precipitation procedure was used. The first stage is the preparation of bentonite solution by the addition of 2 g Na5P3O10 as a dispersing agent to 20 g bentonite ore with the addition of DDW to reach 200 mL with constant stirring for 120 min. The second stage is the preparation of 0.5 M Na2MoO4·2H2O and NaVO3 solutions by dissolving 12.1 g Na2MoO4·2H2O with 100 mL double distilled water and 6.097 g NaVO3 with 100 mL DDW. The third stage is where equimolar solutions (0.5 M) of Na2MoO4·2H2O and NaVO3 were added to bentonite solution with various volumetric ratios as illustrated in Table 1, after the component was added and stirred consistently for 2 h. After that, 10% ammonia solution was slowly added to mixtures with stirring to form a gel at a pH ranging from 7.5 to 8. Then, the reaction mixture was left undisturbed overnight, was washed several times with double distilled water, and was then dried at 60 °C. The resulting solid was converted into an H+ form by mixing it with 0.1 M HNO3 for 24 h, followed by filtering and multiple washed with double distilled water to remove the excess HNO3 and dried at 60 °C. Figure 1 demonstrates the prepared MoV@bentonite composite and ordinary bentonite. Transparent bentonite powder (yellow color) converted to brownish red color after the immobilization of MoV into the surface of the bentonite.
Preliminary studies
The batch technique was used to study the sorption of Cs(I) ions onto bentonite or MoV or MoV@bentonite composites. Typically, 5 mL Cs(I) solution (50 mg·g−1) was added to 0.05 g of the synthesized composites and then shaken in a flask shaker for 24 h at 25 ± 1 °C. Hydrochloric acid and sodium hydroxide were used to adjust the pH of the solution. Finally, the radioactivity of the solution was measured and three replicates were prepared in each case. Equation (1) can be used to calculate the elimination efficiency (E%) (Hamed et al. 2019; Abass et al. 2021):
where Ai and Af are the initial and final activity of 134Cs.
Characterization
Fourier transform infrared (FT-IR) measurement was performed on a Nicolet is10 spectrometer (Meslo, USA) using KBr pellets for sample preparation. X-ray diffraction (XRD) was carried out by a Shimadzu XD-D1, X-ray diffractometer with Cu-Kα radiation tube source (λ = 0.15406 nm) and graphite monochromator operational at 30 kV and 30 mA. A scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) analysis model (ZEISS-EVO 15, UK) were utilized to examine the morphology and elemental analysis of the MoV@bentonite composite.
Chemical stability study
The chemical stability of the MoV@bentonite composite was studied using different solvents (double distilled water, acetone, cyclohexanone, HNO3, HCl, and NaOH). Different solvents (20 mL) were added to MoV@bentonite composite (0.1 g) with constant agitation for about 3 days at room temperature. The amount of MoV@bentonite composite left in the solution was detected gravimetrically (Ibrahim et al. 2021; Kasem et al. 2021; Abass et al. 2022b, c).
Batch studies
Different parameters such as pH (1–12), metal ion concentrations from 25 to 600 mg/L, sample weight (0.009–0.15 g), temperatures (298–338 K), and shaking time from 5 to 4320 min are examined carefully to obtain the optimum conditions for the sorption process. MoV@bentonite composite and Cs(I) ion solution were agitated batchwise till it reached equilibrium; the system was centrifuged at 6000 rpm to separate the liquid phase from the solid phase. In a shaker thermostat model (Kottermann D-1362, Germany), all equilibrium experiments were conducted by contacting 0.05 g of MoV@bentonite composite with 5 mL of Cs(I) ions of the initial concentration (Co) = 50 mg·g−1 with V/m = 0.1 L·g−1. The amount sorbed qe (mg·g−1) was calculated from Eq. (2) (Metwally et al. 2019):
where Co and Ce are the initial and equilibrium concentrations of Cs(I) ions in solution, V is the solution volume (L), and m is MoV@bentonite composite weight (g).
The distribution coefficients (Kd) were computed from Eq. (3) (Şenol and Şimşek 2022):
Saturation capacity
The saturation capacity (qe) of bentonite and MoV@bentonite composites against Cs(I) ions was done by repeated equilibration of 50 mg·L–1 of Cs(I) ions at initial pH 12 with 0.05 g of adsorbent in a flask shaker at 25 ± 2 °C for 2 h. Each equilibration was continued for 5 h. The supernatant liquids were withdrawn, measured, and replaced by an equal volume of the original solution of respective ions. The equilibration was repeated until no further sorption of Cs(I) ions took place on synthesized sorbents. The saturated capacity was computed from Eq. (4).
where n is the sum number of times added new volumes.
Desorption experiments
The desorption experiments were carried out to evaluate the efficiency of different eluents to release Cs(I) ions loaded on the synthesized composite. Desorption of Cs(I) ions from loaded MoV@bentonite was studied by using different eluents. The used eluents are CaCl2, HCl, and EDTA. A series of 50 mL bottles each containing 0.05 g of loaded MoV@bentonite by Cs(I) ions (50 mg·g−1) and 5 mL of (0.03, 0.05, and 0.1 M) from one of these eluents was shaken for 5 h. Finally, the suspensions were centrifuged and then analyzed in the supernatant (Cs) and the solid phase (Cd) (mg·g−1). The desorption percent was determined using Eq. (5) (Dakroury et al. 2021):
Results and Discussion
Preliminary studies
The elimination efficiency of the studied radiotracer either onto bentonite or MoV or MoV@bentonite composites is presented in Table 1. Results reveal that the impregnation of MoV onto bentonite clay increases the elimination efficiency of MoV@bentonite (93.6%) than bentonite (82.6%) and MoV (81.5%); these results are due to increasing the number of active sites of MoV@bentonite leading to increasing in sorption behavior of 134Cs and MoV@bentonite which is the best adsorbent with the highest elimination efficiency of 93.6%.
FT-IR analysis
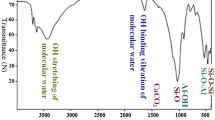
FT-IR spectrum of MoV@bentonite before and after sorption of Cs(I) ions is shown in Fig. 2(a). The observed two bands located at 3437 and 1636 cm−1 can be explained by stretching and bending vibration of OH frequencies of intrastructure water molecules, respectively (Abass et al. 2022c) or attributed to V–OH groups (Rao et al. 2017; Xu et al. 2020). The band observed at 1511 cm−1 corresponds to M–O–H bending vibration mode (Abdel-Galil et al. 2020). Three bands observed at (1042, 792, and 526 cm−1) correspond to Si–O, Si–O-Al, and Si–O-Mg bending, respectively (Mekawy et al. 2022). The V–O bond vibrations in Mo–O–V matrix may be expressed by the FT-IR band at 471 cm–1 (Rao et al. 2017). FT-IR analysis shows that the spectrum of MoV@bentonite either before or after sorption of Cs(I) ions is approximately the same with a very slight shift except for the peak observed at 479 cm−1 which confirms the sorption of Cs(I) ions onto the surface of MoV@bentonite composite as seen later in EDX data. Moreover, the intensity of the band at 1042 cm−1 was increased which confirms the successful loading of Cs(I) ions onto the surface of the MoV@bentonite composite.
XRD analysis
X-ray diffraction pattern of MoV@bentonite is exposed in Fig. 2(b). Several sharp peaks were located at 2Ɵ of (20.3°, 21.05°, 26.82°, 35.5°, and 50.1°) which confirms the crystalline nature of MoV@bentonite with a monoclinic system, where the detected peaks represent the Miller index indications (102, 201, 011, 400, and 412), respectively. These data are in agreement with the XRD of the BPN composite prepared by Mahrous et al. (Mahrous et al. 2022).
SEM and EDX
Figure 3 represents the morphology of the synthesized MoV@bentonite composite before and after the sorption of cesium ions. Figure 3(a) reveals the existence of a considerable number of small cracks and cavities which are filled with Cs(I) ions in Fig. 2(b). The elemental analysis of MoV@bentonite composite before and after sorption of Cs(I) ions is represented in Fig. 2. Results show that the percent of all the components is approximately the same except the percent of molybdenum which is reduced to be 1.2% instead of 3.72%; this reflects the exchange between Mo and Cs inside the structure of MoV@bentonite composite. This confirms that the modification of bentonite with molybdenum vanadate enhances the sorption properties of Cs(I) ions on it. This confirms that cesium ions can be sorbed either inside the cavities and cracks or exchanged with molybdenum.
Chemical stability
The solubility test for MoV@bentonite toward different solvents (Table 2) indicated that the MoV@bentonite was very stable in different solvents which are mentioned in the experimental part. The data represented in Table 2 exhibit that MoV@bentonite composite has good chemical stability compared to other composites (Abdel-Galil et al. 2020, 2021; Ibrahim et al. 2021).
Optimization of batch studies
Influence of pH and point of zero charges
The adsorption process is highly affected by the solution pH, and the solution pH plays an important role in the adsorption process (Şenol and Şimşek 2022). Figure 4(a) shows the variation of the E% and equilibrium sorption capacity of Cs(I) ions onto MoV@bentonite composite, as a function of pH. The experiment was done at fixed temperature of 298 ± 1 K, Co = 50 mg·g−1, V/m = 0.1 L·g−1, agitating time (300 min), and different pHs (1–12). It is evident that the values of E% and equilibrium sorption capacity of Cs(I) ions increase as the pH increases and it is seen that adsorption increases sharply as the pH increases from 2 to 6 and slightly increases from 8 to 12. It is seen that Cs(I) ion removal is lower at acidic pHs. This is because H+ ions and cationic Cs(I) ions compete to adsorb onto MoV@bentonite composite active centers. As the solution pH increases, the electrostatic repulsion forces between the Cs(I) ions and the MoV@bentonite composite surface decrease, increasing Cs(I) ion adsorption (Şenol and Şimşek 2022). At basic pHs, the surface of the MoV@bentonite composite is negatively charged and H-bonds and van der Waals interactions can be seen between the Cs(I) ions and the MoV@bentonite composite, so adsorption is seen to be high. The maximum E% was done at pH 12 with no change above this pH, and all experiments were done at this value.
The solution pH value at which the surface charge of the adsorbent is zero is defined as the point of zero charges (PZC). To determine the PZC value of the MoV@bentonite composite, the MoV@bentonite composite was kept in solutions containing 0.1 mol L−1 NaNO3 in the pH = 1–12 range for 24 h, and the equilibrium pH was measured. 0.1 M HCl or NaOH was used to adjust the pH. The PZC value was obtained from the linear relationship between the initial pHi and ΔpH. The ΔpH value was obtained from the difference between the initial and final pH (ΔpH = pHf − pHi). The surface charge of the MoV@bentonite composite was found to be 8.98 (Fig. 4(b)). The surface of the MoV@bentonite composite was positive (pH < pHpzc) below pH 8.98 and negative (pH > pHpzc) above pH 8.98. The condition pH > pHpzc explains the increase.
Influence of shaking time
At fixed temperature (298 ± 1 K), initial concentration (Co) = 50 mg·g−1, V/m = 0.1 L.g−1, shaking time (5–4320 min), and pH = 12, the effect of shaking time on the elimination efficiency (E%) of Cs(I) ions onto MoV@bentonite composite is exposed in Fig. 5(a). It was found that the E% of Cs(I) ions onto MoV@bentonite composite very rapidly increases with time (5–180 min) and slowly increases (180–300 min), and there is no change of the E% after this time, so 300 min was used as equilibrium time.
Effect of MoV@bentonite dose
Fixed volume and different weights of MoV@bentonite composite were used to determine the minimal weight of MoV@bentonite composite to provide a reasonable E% of Cs(I) ions at a constant concentration. The weight of the synthesized composite was increased from 0.009 to 0.15 g, while the volume of the aqueous solution was fixed (5 mL). The effect of the MoV@bentonite weight on the E% of Cs(I) ions is shown in Fig. 5(b). The observations show that an increase in the E% occurs with the corresponding increases in the MoV@bentonite weight. The increases in the E% are due to the increase in its functional groups, and more active sites were available (Hamed et al. 2016b). As seen in Fig. 5(b), the E% of Cs(I) ions increased slowly from 44.8% to 58.1% when the MoV@bentonite weight was increased from 0.009 to approximately 0.03 g, whereas the sorption of Cs(I) ions increased quickly (58.1% to 95.93%) when the MoV@bentonite weight was changed from 0.03 to 0.05 g and then slightly (95.93% to 99.89%) when the MoV@bentonite weight increased from 0.05 g to 0.15 g.
Effect of ionic strength
Figure 5(c) shows the plots between the E% and sorption capacity of Cs(I) ions onto MoV@bentonite composite and the ionic strength of NaCl (0.02–0.3 M). The experiment was performed at Co = 50 mg·g−1, V/m = 0.1 L·g−1, agitating time 300 min, and pH = 12. Figure 5(c) shows a great reduction in the E% of Cs(I) ions with increasing molar concentration of NaCl from 0.02 to 0.2 M. The E% of Cs(I) ions decreased from 82.69% to 51.15% with rising NaCl concentration up to 0.2 M. This reduction could be referred to as the presence of Na(I) retarded the movement of Cs(I) ions from bulk solution towards the surface of the MoV@bentonite composite. Ion exchange or outer-sphere surface complexation is primarily responsible for the strong ionic strength-dependent sorption (Sheha et al. 2020).
Saturation capacity
The saturation capacity of Cs(I) ions on bentonite and MoV@bentonite composites was determined at 298 ± 1 K and pH 12 and recorded that 18.92 and 26.72 mg·g−1, respectively. The saturation capacity reveals that MoV@bentonite composite has a higher capacity value for Cs(I) ions than bentonite composite reflecting a good modification by adding MoV to bentonite composite as well as the MoV@bentonite composite has a higher capacity value for Cs(I) ions compared with inorganic composites (Yi et al. 2014; Jang et al. 2015; Hamed et al. 2016a; Abdel-Galil et al. 2019; Ke et al. 2020). Table 3 compares the capacity of MoV@bentonite composite with that of other composites for Cs(I) ions found in the literature. The prepared MoV@bentonite composite can be observed to have a higher capacity than many other sorbents. As a result, it can be seen that MoV@bentonite composite is a promising composite for removing Cs(I) ions from aqueous solutions.
Kinetic study
Some kinetic equations such as pseudo-first-order (PFO), pseudo-second-order (PSO), and intra-particle diffusion model (Weber-Morris) (IPD) were used to check the experimental data and can be obtained from the next equations (Dao et al. 2022; Kumari et al. 2022; Şimşek et al. 2022):
Here, qt (mg·g−1) is the value of the amount sorbed per unit mass at any time t. K1 (min−1), K2 (g·mg−1·min−1), and KIPD (mg/g·min0.5) are the rate constants of the PFO, PSO, and IPD, respectively. The plotted nonlinear forms of PFO and PSO are present in Fig. 6(a and b), respectively as well as the linear fitting form (IPD) as shown in Fig. 6(c). The K1, K2, and KIPD constants as well as the correlation coefficient (R2) derived from these plots are presented in Table 4. In the first stage, rapid adsorption took place, involving the first 60 min. In the second phase (from 120 to 240 min), there was longer slower adsorption, possibly involving the interior of the adsorbent. The third stage from 300 to 4320 min includes the equilibrium saturation. The first stage was fast and quantitatively dominant, and the second stage was slower and quantitatively insignificant. During the initial stage of adsorption, there were many available active sites on the MoV@bentonite surface. After these sites were occupied, the equilibrium state was realized, and the second stage was started, involving the inner regions of the adsorbent. The rapid stage results from the abundance of active sites on the MoV@bentonite composite surface, while the gradual filling of these sites also makes the adsorption process less efficient during the slower stage. In the first stage of adsorption, there are many active sites on the MoV@bentonite composite surface. Cs(I) ions are adsorbed to these active sites. Over time, the number of active sites on the MoV@bentonite composite surface decreases, and the Cs(I) ions become saturated. In the next step, the Cs(I) ions diffuse slowly through the pore of the MoV@bentonite composite. Therefore, adsorption slows down. When the correlation coefficients (R2) of the PFO and PSO models were compared with each other, it was seen that the results fit the PSO kinetic model better. In addition, the closeness of the theoretically calculated qt and experimental qe values showed compatibility with the PSO model. These results showed that the adsorption process followed the PSO rate kinetics. Furthermore, the multilinear curves seen in the IPD plot indicated that the adsorption process involved two or more steps, as highlighted above. In this case, it was shown that it is not possible to explain the adsorption with a single kinetic model. Cs(I) ions first rapidly filled active sites on the surface of the MoV@bentonite composite and then diffused slowly and gradually through the pore of the MoV@bentonite composite. The adsorption process is accompanied by surface diffusion, film diffusion, diffusion on the pore surface, or more than one of these steps.
Effect of ion concentration
Figure 7(a) shows the plots between both E% and qe (mg·g−1) of Cs(I) ions onto MoV@bentonite composite and the initial concentrations of Cs(I) ions. The experiment was performed at Co = 25–600 mg/L, V/m = 0.1 L.g−1, agitating time of 300 min, and pH = 12. Figure 7(a) displays a great reduction in the E % of Cs(I) ions with increasing metal concentrations, and the maximum E % of Cs(I) ions was achieved at initial concentrations of 50 mg·g−1, whereas the amount uptake (mg·g−1) of Cs(I) ions increases with increasing initial concentration.
Sorption isotherm models
The concentration data obtained to determine the isotherms of the sorption of Cs(I) ions by MoV@bentonite composite were analyzed using Langmuir isotherm [Eq. (9)] (Chen et al. 2022), Freundlich isotherm [Eq. (10)] (Chang and Juang 2004), and Dubinin-Radushkevich (D-R) isotherm [Eqs. (11–13)] (Şenol et al. 2022) models. The Langmuir isotherm model assumes that the active sites where adsorption that took place is homogeneously distributed on the adsorbent surface (Langmuir 1918). The Freundlich isotherm model explains a hyperbolic adsorption behavior and gives information about the heterogeneity of the adsorbent surface (Freundlich 1907). The D-R isotherm model assumes that adsorption is related to surface porosity and pore volume. The D-R isotherm model examines adsorption from an energetic point of view and specifies the adsorption process physically or chemically. If the EDR value is in the range of 8–16 kJ mol−1, the adsorption process is chemical; if the EDR is < 8 kj mol−1, the adsorption process is physical. The EDR value for this study was within the range of 8–16 kjmol−1 (Şenol et al. 2022).
Qmax (mg·g−1) is the simulated monolayer capacity, and b (L/mg) is the Langmuir equilibrium constant related to the energy of sorption and describes the affinity of binding sites, KF is Freundlich constant, \(1/n\) is the adsorbent surface heterogeneity, qmax (mg·g−1) is a measure of adsorption capacity, KDR and ɛ is based on sorption energy (Polanyi potential, kJ/mol), R is the ideal gas constant (8.314 Jmol−1 K−1), EDR is the adsorption energy (kJ mol− 1), and T is the absolute temperature (K).
Adsorption isotherms were used to establish the interaction mechanism between adsorbent and Cs(I) ions at equilibrium. Comparing the R2 values derived from the Langmuir and Freundlich isotherm models (Fig. 7(b and c), Table 5), it can be seen that the adsorption process conformed to both Langmuir and Freundlich isotherm models. Sorption of Cs(I) ions onto MoV@bentonite composite provided a better fit with the Freundlich model (R2 = 0.96). KF, which is a measure of adsorption capacity, was 4.89, and 1/n surface heterogeneity was 0.26, from the Freundlich isotherm model. The 1/n surface heterogeneity showed that the conditions were favorable for the adsorption. The maximum adsorption capacity Qmax (mg·g−1) was 22.06 mg/g, and the b value was 0.04 L/mg. The high adsorption capacity of MoV@bentonite composite is promising for its use as an adsorbent for the removal of Cs(I) ions from aqueous solutions. The magnitude of the EDR value in the D-R model characterizes the type of adsorption process (Fig. 7(d)). The adsorption energy, 14.21 kJ mol−1, derived by the D-R model, suggested that the nature of the adsorption is chemical.
Thermodynamic studies
The influence of temperature on the E% of Cs(I) ions by MoV@bentonite composite is represented in Fig. 8(a). This figure illustrates how the endothermic nature of the sorption process is reflected by an increase in the E% of Cs(I) ions with increasing reaction temperature. The linear relationship between ln Kd of Cs(I) ions on MoV@bentonite composite and 1000/T through the Van't Hoff relation is shown in Fig. 7(b) (Abdel-Galil et al. 2016);
Here, \(\Delta H^\circ , \Delta S^\circ , R, \mathrm{and}\;T\) are the changes of enthalpy (kJ/mol), change of entropy (J/mol·K), gas constant (J.K−1·mol−1), and absolute temperature (K). The Kd of Cs(I) ions improved with increasing reaction temperature from 298 to 338 °K. From both slopes and intercepts of linear relation shown in Fig. 7(b), \(\Delta H^\circ\) and \(\Delta S^\circ\) were computed and existing in Table 6. The positive values of both \(\Delta H^\circ\) and \(\Delta S^\circ\) reflect the endothermic nature and increased randomness of the solid solution interface through the adsorption of Cs(I) ions on MoV@bentonite composite, respectively (Abass et al. 2022c). The free energy change of adsorption ∆G° (kJ/mol) was obtained by the relation:
The negative values of ∆G° displayed in Table 6 reflect that the sorption process is spontaneous and indicates the better sorption of Cs(I) ions on MoV@bentonite composite compared with H+ ion (Abass et al. 2022c). By comparison of these data in the present work with other data from the literature, it is clear that ∆H° and ∆S° for Cs(I) sorbed onto MoV@bentonite composite have higher values than P(AN-AM)-NS and ZrSnP prepared by Abass et al. (Abass et al. 2022b, c) and lower than SnV prepared by Abass et al. (Abass et al. 2022a). And ∆G° for Cs(I) ions sorbed onto MoV@bentonite composite has lower values than P(AN-AM)-NS and ZrSnP prepared by Abass et al. (Abass et al. 2022b, c) and higher than SnV prepared by Abass et al. (Abass et al. 2022a). This comparison proves that the MoV@bentonite considers a promised composite for the sorption of Cs(I) ions.
Adsorption mechanism
The mechanism of adsorption of Cs(I) ions onto the MoV@bentonite composite can be explained by the strong attraction forces of functional groups on the composite surface as shown in Scheme 1. The functional groups on the surface of the MoV@bentonite composite were mainly such as Mo–O, Mg-O, and hydroxyl groups (confirmed by the FT-IR spectrum). It could be very likely that electrostatic attraction plays an important role in the adsorption of Cs(I) ions. For a general mechanism, the following explanation was suggested: (i) migration of the Cs(I) ions onto the surface of the MoV@bentonite composite (bulk solution transport), (ii) diffusion of the Cs(I) ions across the boundary layer to the surface of the MoV@bentonite composite (film diffusion), and (iii) adsorption of the Cs(I) ions onto the surface of the MoV@bentonite composite. Mo–O, Mg-O, hydroxyl groups, and O bridges on the surface of MoV@bentonite composite form adsorption sites. The Mo–O and Mg-O may be exchanged with Cs(I) ions as seen in EDX results. The –OH groups of the MoV@bentonite composite could form H-bonds with the Cs(I) ions and function as adsorption sites. The final stage of the adsorption process could involve the penetration of some Cs(I) ions into the MoV@bentonite composite via intra-particle diffusion. In light of this information, it is thought that the adsorption process of Cs(I) ions in the MoV@bentonite composite are accompanied by surface diffusion, film diffusion, intraparticle diffusion, and diffusion on the pore surface.
Desorption studies
The desorption studies were carried out by eluting Cs(I) ions from loaded MoV@bentonite composite using different eluting agents such as CaCl2, HCl, and EDTA with different concentrations (0.03, 0.05, and 0.1 M), and the results are depicted in Fig. 9(a). Data illustrate that the recovery of studied radionuclide increase with increasing eluents concentration, and the best eluent is HCl (76.9%) at 0.1 M. The sequence order for the % desorption process is HCl ˃ CaCl2 ˃ EDTA.
Recycling study
The repeated use of MoV@bentonite composite is an essential feature where the subsequent regeneration of the sorbent for another cycle of the application is desirable. The recycling efficiency of MoV@bentonite composite and its potential use as a solid phase was done by successive adsorption and desorption cycles of Cs(I) ions at the optimal condition. MoV@bentonite composite loaded with Cs(I) ions was recycled using 0.1 M HCl as an eluent. This eluent is used because it attained the highest desorption percentage of Cs(I) ions from the loaded-on MoV@bentonite composite in the desorption experiment compared with the other used eluents. The synthesized MoV@bentonite composite was used in repeated sorption–desorption cycles. Figure 9(b) illustrates the relationship between the E% and the cycle number. The result illustrated that the synthesized MoV@bentonite composite could be reused for sorption–desorption cycles up to 7 cycles with a slight decrease in the E% from cycle one to cycle two also, the E% slightly decreased from cycle two to cycle three, and so on. The excellent regeneration argues the applicability of MoV@bentonite to be used repeatedly as an effective material for the sorption of Cs(I) ions from aqueous media with very good efficiencies.
Conclusion
In this article, the MoV@bentonite composite was successfully synthesized through the precipitation method and tested for its performance in the sorption of Cs(I) ions from aqueous solutions. The sorption experiments of Cs(I) ions confirm that the prepared composite has an equilibrium time of (300 min), applicable to the Langmuir and Freundlich isotherm models, and more fitted to the pseudo-second-order kinetic model. Experiments show that the sorption of Cs(I) ions onto MoV@bentonite composite is dependent on pH values and the optimum pH value is pH = 12. Saturation capacity experiments illustrate that the maximum Cs(I) ions sorbed onto MoV@bentonite composite is 26.72 mg·g−1. Thermodynamic parameters were endothermic and spontaneous. 0.1 M HCl shows optimal desorption of Cs(I) ions from an aqueous solution. All the previous data show that MoV@bentonite composite has several advantages such as low cost, high reliability, and high performance for decontamination of cesium-134 while the chief disadvantage is that the equilibrium was reached within 300 min. Finally, the high sorption efficiency of the MoV@bentonite composite suggests that it can be used as an effective and promising sorbent for Cs(I) ions from aqueous solutions in the future.
Data availability
Yes.
References
Abass MR, Ibrahim AB, El-Masry EH, Abou-Mesalam MM (2021) Optical properties enhancement for polyacrylonitrile-ball clay nanocomposite by heavy metals saturation technique. J Radioanal Nucl Chem 329:849–855. https://doi.org/10.1007/s10967-021-07844-3
Abass MR, Breky MME, Maree RM (2022a) Removal of 137Cs and 90Sr from simulated low-level radioactive waste using tin(IV) vanadate sorbent and its potential hazardous parameters. Appl Radiat Isot 189:110417
Abass MR, El-Masry E, El-Kenany WM (2022b) Gamma irradiation-induced preparation of polyacrylonitrile acrylamide nano-silica for removal of some hazardous metals. J Inorg Organomet Polym Mater 32:536–546. https://doi.org/10.1007/s10904-021-02156-1
Abass MR, Maree RM, Sami NM (2022c) Adsorptive features of cesium and strontium ions on zirconium tin(IV) phosphate nanocomposite from aqueous solutions. Int J Environ Anal Chem:1–20. https://doi.org/10.1080/03067319.2021.2016728
Abdel Rahman RO, Abdel Moamen OA, Abdelmonem N, Ismail IM (2019) Optimizing the removal of strontium and cesium ions from binary solutions on magnetic nano-zeolite using response surface methodology (RSM) and artificial neural network (ANN). Environ Res 173:397–410. https://doi.org/10.1016/j.envres.2019.03.055
Abdel-Galil EA, Ibrahim AB, Abou-Mesalam MM (2016) Sorption behavior of some lanthanides on polyacrylamide stannic molybdophosphate as organic-inorganic composite. Int J Ind Chem 7:231–240
Abdel-Galil EA, Hassan RS, Eid MA (2019) Assessment of nano-sized stannic silicomolybdate for the removal of 137Cs, 90Sr, and 141Ce radionuclides from radioactive waste solutions. Appl Radiat Isot 148:91–101
Abdel-Galil EA, Eid MA, Hassan RS (2020) Preparation of nanosized stannic silicomolybdate for chromatographic separation of Y(III) from Zr(IV). Part Sci Technol 38:113–120
Abdel-Galil EA, Ibrahim AB, El-Kenany WM (2021) Facile fabrication of a novel silico vanadate ion exchanger: evaluation of its sorption behavior towards europium and terbium ions. Desalin Water Treat 226:303–318. https://doi.org/10.5004/dwt.2021.27261
Ahn JM, Kim S, Kim Y-S (2020) Selection of priority management of rivers by assessing heavy metal pollution and ecological risk of surface sediments. Environ Geochem Health 42:1657–1669
Andrunik M, Bajda T (2019) Modification of bentonite with cationic and nonionic surfactants: structural and textural features. Materials (Basel) 12:3772
Belousov P, Semenkova A, Egorova T et al (2019) Cesium sorption and desorption on glauconite, bentonite, zeolite, and diatomite. Minerals 9:625
Brigatti MF, Galán E, Theng BKG (2013) Structure and mineralogy of clay minerals. Dev Clay Sci 5:21–81
Chang M, Juang R (2004) Adsorption of tannic acid , humic acid , and dyes from water using the composite of chitosan and activated clay. 278:18–25. https://doi.org/10.1016/j.jcis.2004.05.029
Chen Z, He X, Li Q et al (2022) Low-temperature plasma induced phosphate groups onto coffee residue-derived porous carbon for efficient U(VI) extraction. J Environ Sci 122:1–13. https://doi.org/10.1016/j.jes.2021.10.003
Dakroury GA, El-Shazly EAA, Hassan HS (2021) Preparation and characterization of ZnO/Chitosan nanocomposite for Cs(I) and Sr(II) sorption from aqueous solutions. J Radioanal Nucl Chem 330:159–174
Dao PT, Tran NYT, Tran QN et al (2022) Kinetics of pilot-scale essential oil extraction from pomelo (Citrus maxima) peels: comparison between linear and nonlinear models. Alexandria Eng J 61:2564–2572. https://doi.org/10.1016/j.aej.2021.07.002
Dinh VP, Nguyen PT, Tran MC et al (2022) HTDMA-modified bentonite clay for effective removal of Pb(II) from aqueous solution. Chemosphere 286:131766. https://doi.org/10.1016/j.chemosphere.2021.131766
Donat R, Akdogan A, Erdem E, Cetisli H (2005) Thermodynamics of Pb2+ and Ni2+ adsorption onto natural bentonite from aqueous solutions. 286:43–52. https://doi.org/10.1016/j.jcis.2005.01.045
El-Din AFT, Elshehy EA, Abd El-Magied MO et al (2018) Decontamination of radioactive cesium ions using ordered mesoporous monetite. RSC Adv 8:19041–19050
Freundlich H (1907) Über die adsorption in lösungen. Zeitschrift Für Phys Chemie 57:385–470
Fuller AJ, Shaw S, Peacock CL et al (2014) Ionic strength and pH dependent multi-site sorption of Cs onto a micaceous aquifer sediment. Appl Geochem 40:32–42
Ghaly M, El-Sherief EA, Metwally SS, Saad EA (2018) Utilization of nano-cryptomelane for the removal of cobalt, cesium and lead ions from multicomponent system: kinetic and equilibrium studies. J Hazard Mater 352:1–16. https://doi.org/10.1016/j.jhazmat.2018.03.020
Ghaly M, Metwally SS, El-Sherief EA, et al (2022) Utilization of synthetic nano-cryptomelane for enhanced scavenging of cesium and cobalt ions from single and binary solutions. J Radioanal Nucl Chem 1–18
Hamed MM, Holiel M, Ahmed IM (2016a) Sorption behavior of cesium, cobalt and europium radionuclides onto hydroxyl magnesium silicate. Radiochim Acta 104:873–890
Hamed MM, Rizk SE, Nayl AA (2016b) Adsorption kinetics and modeling of gadolinium and cobalt ions sorption by an ion-exchange resin. Part Sci Technol 34:716–724
Hamed MM, Shahr El-Din AM, Abdel-Galil EA (2019) Nanocomposite of polyaniline functionalized Tafla: synthesis, characterization, and application as a novel sorbent for selective removal of Fe(III). J Radioanal Nucl Chem 322:663–676. https://doi.org/10.1007/s10967-019-06733-0
Huang R, Lin Q, Zhong Q et al (2020) Removal of Cd(II) and Pb(II) from aqueous solution by modified attapulgite clay. Arab J Chem 13:4994–5008. https://doi.org/10.1016/j.arabjc.2020.01.022
Ibrahim AB, Abass MR, EL-Masry EH, Abou-Mesalam MM (2021) Gamma radiation-induced polymerization of polyacrylic acid-dolomite composite and applications for removal of cesium, cobalt, and zirconium from aqueous solutions. Appl Radiat Isot 178:109956
Ivanets A, Milyutin V, Shashkova I et al (2020) Sorption of stable and radioactive Cs(I), Sr(II), Co(II) ions on Ti–Ca–Mg phosphates. J Radioanal Nucl Chem 324:1115–1123
Iwai T, Hashimoto Y (2017) Adsorption of tungstate (WO4) on birnessite, ferrihydrite, gibbsite, goethite and montmorillonite as affected by pH and competitive phosphate (PO4) and molybdate (MoO4) oxyanions. Appl Clay Sci 143:372–377. https://doi.org/10.1016/j.clay.2017.04.009
Izosimova Y, Gurova I, Tolpeshta I et al (2022) Adsorption of Cs(I) and Sr(II) on bentonites with different compositions at different pH. Minerals 12:862
Jang S-C, Haldorai Y, Lee G-W et al (2015) Porous three-dimensional graphene foam/Prussian blue composite for efficient removal of radioactive 137Cs. Sci Rep 5:17510
Karthik R, Meenakshi S (2015) Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem Eng J 263:168–177
Kasem AE, Abdel-Galil EA, Belacy N, Badawy NA (2021) Kinetics and adsorption equilibrium of some radionuclides on polyaniline/SiO2 composite. Radiochim Acta 109:85–97
Ke Y, Li Y, Zhu L et al (2020) Rapid enrichment of cesium ions in aqueous solution by copper ferrocyanide powder. SN Appl Sci 2:522–532
Kumari B, Tiwary RK, Yadav M (2022) Non linear regression analysis and response surface modeling for Cr(VI) removal from aqueous solution using poly-aniline coated sugarcane bagasse (PANI@SB) composites as an adsorbent. Surf Interfaces 29:101729. https://doi.org/10.1016/j.surfin.2022.101729
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lin M, Kajan I, Schumann D et al (2020) Selective Cs-removal from highly acidic spent nuclear fuel solutions. Radiochim Acta 108:615–626. https://doi.org/10.1515/ract-2019-3187
Mahrous SS, Abass MR, Mansy MS (2022) Bentonite phosphate modified with nickel: preparation, characterization, and application in the removal of 137Cs and 152+154Eu. Appl Radiat Isot 190:110445
Mekawy ZA, El Shazly EAA, Mahmoud MR (2022) Utilization of bentonite as a low-cost adsorbent for removal of 95Zr(IV), 181Hf(IV) and 95Nb(V) radionuclides from aqueous solutions. J Radioanal Nucl Chem 331(9):3935–3948
Metwally SS, Hassan HS, Samy NM (2019) Impact of environmental conditions on the sorption behavior of 60Co and 152+154Eu radionuclides onto polyaniline/zirconium aluminate composite. J Mol Liq 287:110941
Noli F, Fedorcea V, Misaelides P et al (2021) Cesium and barium removal from aqueous solutions in the presence of humic acid and competing cations by a Greek bentonite from Kimolos Island. Appl Radiat Isot 170:109600. https://doi.org/10.1016/j.apradiso.2021.109600
Olu-Owolabi BI, Unuabonah EI (2011) Adsorption of Zn2+ and Cu2+ onto sulphate and phosphate-modified bentonite. Appl Clay Sci 51:170–173. https://doi.org/10.1016/j.clay.2010.10.022
Rao KTV, Souzanchi S, Yuan Z et al (2017) Simple and green route for preparation of tin phosphate catalysts by solid-state grinding for dehydration of glucose to 5-hydroxymethylfurfural (HMF). RSC Adv 7:48501–48511. https://doi.org/10.1039/c7ra10083c
Sankararamakrishnan N, Jaiswal M, Verma N (2014) Composite nanofloral clusters of carbon nanotubes and activated alumina: an efficient sorbent for heavy metal removal. Chem Eng J 235:1–9
Şenol ZM, Şimşek S (2022) Insights into effective adsorption of lead ions from aqueous solutions by using chitosan-bentonite composite beads. J Polym Environ 30:3677–3687
Şenol ZM, Kaya S, Şimşek S et al (2022) Synthesis and characterization of chitosan-vermiculite-lignin ternary composite as an adsorbent for effective removal of uranyl ions from aqueous solution: experimental and theoretical analyses. Int J Biol Macromol 209:1234–1247
Sheha RR, Mekawy ZA, Someda HH et al (2020) Assessing the sorptive ability of synthesized graphene oxide-metal oxide composite to remove certain lanthanides. CLEAN–Soil, Air, Water 48:2000348
Şimşek S, Kaya S, Şenol ZM et al (2022) Theoretical and experimental insights about the adsorption of uranyl ion on a new designed Vermiculite-Polymer composite. J Mol Liq 352:118727
Veliscek-Carolan J, Jolliffe KA, Hanley TL (2013) Selective sorption of actinides by titania nanoparticles covalently functionalized with simple organic ligands. ACS Appl Mater Interfaces 5:11984–11994
Wahab N, Saeed M, Ibrahim M et al (2019) Synthesis, characterization, and applications of silk/bentonite clay composite for heavy metal removal from aqueous solution. Front Chem 7:654. https://doi.org/10.3389/fchem.2019.00654
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456. https://doi.org/10.1016/j.carbpol.2010.11.004
Xu Y, Zhou F, Chen M et al (2020) Facile assembly of 2D α-zirconium phosphate supported silver nanoparticles: superior and recyclable catalysis. New J Chem 44:9793–9801. https://doi.org/10.1039/d0nj01378a
Yang S, Han C, Wang X, Nagatsu M (2014) Characteristics of cesium ion sorption from aqueous solution on bentonite-and carbon nanotube-based composites. J Hazard Mater 274:46–52
Yang J, Tai W, Wu F et al (2022) Enhanced removal of radioactive iodine anions from wastewater using modified bentonite: experimental and theoretical study. Chemosphere 292:133401. https://doi.org/10.1016/j.chemosphere.2021.133401
Yi R, Ye G, Wu F et al (2014) Highly efficient removal of 137Cs in seawater by potassium titanium ferrocyanide functionalized magnetic microspheres with multilayer core-shell structure. RSC Adv 4:37600–37608
Zhang H, Zhou J, Muhammad Y et al (2019) Citric acid modified bentonite for congo red adsorption. Front Mater 6:1–11. https://doi.org/10.3389/fmats.2019.00005
Zhou M, Xu Y, Luo G et al (2022) Removal of elemental mercury from coal combustion flue gas using bentonite modified with Ce-Fe binary oxides. Appl Surf Sci 590:153090
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work has been supported by the Egyptian Atomic Energy Authority.
Author information
Authors and Affiliations
Contributions
M. Ghaly: conceptualization, visualization, experimental work, writing—reviewing, and editing. Mohamed R. Abass: data curation, writing—original draft review, and editing. Zakaria A. Mekawy: experimental work, editing, and reviewing.
Corresponding author
Ethics declarations
Ethics approval
Yes
Consent to participate
Yes
Consent for publication
Yes
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghaly, M., Abass, M.R. & Mekawy, Z.A. Performance of molybdenum vanadate loaded on bentonite for retention of cesium-134 from aqueous solutions. Environ Sci Pollut Res 30, 60432–60446 (2023). https://doi.org/10.1007/s11356-023-26607-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26607-z