Abstract
FeMnMg-LDH with hydrotalcite-like structure was synthesized via a coprecipitation method and applied to copper ion removal from aqueous solution. FT-IR, SEM, PXRD, TEM, and XPS were applied to characterization analysis. Factors like temperature, pH, contact time, initial concentration, and coexisting cations were systematically studied. FeMnMg-LDH has an excellent performance on copper adsorption, with a maximum capacity of 204.07 mg/g at 25 °C, which is much higher than that of most other similar LDHs. The effects of coexisting cations on the removal efficiency of copper ions are various and following the order of Zn2+ > Pb2+ > Mg2+ > Ca2+, which may be due to the solubility of their hydroxides. The result of adsorption thermodynamics indicates that the adsorption process is endothermic and spontaneous. The adsorption kinetics are in accord with the pseudo-second-order kinetic model, and particles that appear in the SEM figures are both suggesting that chemical complex formed during the adsorption process. Meanwhile, the result of DKR model fitting indicates that the isomorphic substitution mechanism is involved in the adsorption process. Thus, the main adsorption mechanisms of FeMnMg-LDH in the removal of Cu2+ from aqueous solution in this study are isomorphic substitution and surface-induced precipitation.

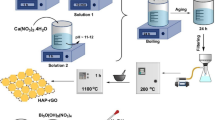
Graphical abstract







Similar content being viewed by others
References
Aoki, A. (1976). X-ray photoelectron spectroscopic studies on ZnS: MnF2 phosphors. Japanese Journal of Applied Physics, 15, 305–311.
Azimi, A., Azari, A., Rezakazemi, M., & Ansarpour, M. (2017). Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Reviews, 4, 37–59.
Ben-Sasson, M., Lu, X., Nejati, S., Jaramillo, H., & Elimelech, M. (2016). In situ surface functionalization of reverse osmosis membranes with biocidal copper nano-particles. Desalination, 388, 1–8.
Carver, J. C., Schweitzer, G. K., & Carlson, T. A. (1972). Use of X-ray photoelectron spectroscopy to study bonding in Cr, Mn, Fe, and Co compounds. The Journal of Chemical Physics, 57, 973–982.
Chen, D., Li, Y., Zhang, J., Zhou, J. Z., Guo, Y., & Liu, H. (2012). Magnetic Fe3O4/ZnCr-layered double hydroxide composite with enhanced adsorption and photo catalytic activity. Chemical Engineering Journal, 185-186, 120–126.
Chen, H., Lin, J. H., Zhang, N., Chen, L. Z., Zhong, S. P., Wang, Y., Zhang, W. G., & Ling, Q. D. (2018). Preparation of MgAl-EDTA-LDH based electrospun nano-fiber membrane and its adsorption properties of copper(II) from wastewater. Journal of Hazardous Materials, 345, 1–9.
Çifci, C., & Şanlı, O. (2010). Crossflow filtration of iron(III), copper(II), and cadmium(II) aqueous solutions with alginic acid/cellulose composite membranes. Journal of Applied Polymer Science, 115, 616–623.
Deroubaix, G., & Marcus, P. (1992). X-ray photoelectron-spectroscopy analysis of copper and zinc-oxides and sulfides. Surface and Interface Analysis, 18, 39–46.
Feng, Y., Gong, J. L., Zeng, G. M., Niu, Q. Y., Zhang, H. Y., Niu, C. G., Deng, J. H., & Yan, M. (2010). Adsorption of Cd(II) and Zn(II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chemical Engineering Journal, 162, 487–494.
František, K., Tomáš, G., & Vít, D. (2003). Thermal behaviour of Ni-Mn layered double hydroxide and characterization of formed oxides. Solid State Sciences, 5, 1019–1026.
González, M. A., Pavlovic, I., Rojas-Delgado, R., & Barriga, C. (2014). Removal of Cu2+, Pb2+ and Cd2+ by layered double hydroxide-humate hybrid, sorbate and sorbent comparative studies. Chemical Engineering Journal, 25, 605–611.
González, M. A., Pavlovic, I., & Barriga, C. (2015). Cu(II), Pb(II) and Cd(II) sorption on different layered double hydroxides. A kinetic and thermodynamic study and competing factors. Chemical Engineering Journal, 269, 221–228.
Hawn, D. D., & DeKoven, B. M. (1987). Deconvolution as a correction for photoelectron inelastic energy losses in the core level XPS spectra of iron oxides. Surface and Interface Analysis, 63, 10–12.
Helfferich, F. (1962). Ion exchange (Vol. 166). New York: McGraw-Hill.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465.
Hu, K., Wang, K., Liu, J., & Dong, Q. (2015). Hybrid membrane adsorbents: preparation and their adsorptions for copper(II) ions. Desalination and Water Treatment, 65, 1–10.
Huang, G., Wang, D., Ma, S., Chen, J., Jiang, L., & Wang, P. (2015). A new, low-cost adsorbent: preparation, characterization, and adsorption behavior of Pb(II) and Cu(II). Journal of Colloid and Interface Science, 445, 294–302.
Li, Y. L., Wang, J., Li, Z. S., Liu, Q., Liu, J. Y., Liu, L. H., Zhang, X. F., & Yu, J. (2013). Ultrasound assisted synthesis of Ca-Al hydrotalcite for U(VI) and Cr(VI) adsorption. Chemical Engineering Journal, 218, 295–302.
Li, R., Wang, J. J., Zhou, B., Awasthi, M. K., & Ali, A. (2016). Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Science of the Total Environment, 559, 121–129.
Liang, X. F., Zang, Y. B., Xu, Y. M., Tan, X., Hou, W. G., Wang, L., & Sun, Y. B. (2013). Sorption of metal cations on layered double hydroxides. Colloids Surface A, 433, 122–131.
McIntyre, N. S., & Zetaruk, D. G. (1977). X-ray photoelectron spectroscopic studies of iron oxides. Analytical Chemistry, 49, 1521–1529.
Park, M., Choi, C. L., Seo, Y. J., Yeo, S. K., Choi, J., Komarneni, S., & Lee, J. H. (2007). Reactions of Cu2+ and Pb2+ with Mg/Al layered double hydroxide. Applied Clay Science, 37, 143–148.
Peng, C., Chai, L. Y., Tang, C. J., Min, X. B., Ali, M., Song, Y. X., & Qi, W. M. (2017). Feasibility and enhancement of copper and ammonia removal from wastewater using struvite formation: a comparative research. Journal of Chemical Technology and Biotechnology, 92, 325–333.
Rojas, R. (2016). Effect of particle size on copper removal by layered double hydroxides. Chemical Engineering Journal, 303, 331–337.
Scarazzato, T., Buzzi, D. C., Bernardes, A. M., & Romano Espinosa, D. C. (2015). Treatment of wastewaters from cyanide-free plating process by electrodialysis. Journal of Cleaner Production, 91, 241–250.
Seida, Y., & Nakano, Y. (2000). Removal of humic substances by layered double hydroxide containing iron. Water Research, 34, 1487–1494.
Stern, B. R. (2010). Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. Journal of Toxicology and Environmental Health. Part A, 73, 114–127.
Sui, M. H., Duan, B. B., Sheng, L., Huang, S. H., & She, L. (2012). Catalytic performance of layered double hydroxides Co-Mn-Al for ozonation of organic pollutants in water. Chinese Journal of Catalysis, 33, 1284–1289.
Tan, B. J., Klabunde, K. J., & Sherwood, P. M. A. (1991). XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. Journal of the American Chemical Society, 113, 855–861.
Tong, D. S., Liu, M., Li, L., Lin, C. X., Yu, W. H., Xu, Z. P., & Zhou, C. H. (2012). Transformation of alunite residuals into layered double hydroxides and oxides for adsorption of acid red G dye. Applied Clay Science, 70, 1–7.
Tran, H. N., et al. (2017). Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Research, 120, 88–116.
Tran, H. N., Lin, C. C., Woo, S. H., & Chao, H. P. (2018). Efficient removal of copper and lead by Mg/Al layered double hydroxides intercalated with organic acid anions: adsorption kinetics, isotherms, and thermodynamics. Applied Clay Science, 154, 17–27.
Umezawa, Y., & Reilley, C. N. (1978). Correlation of Mn charge state with the electrical resistivity of Mn doped indium tin oxide films. Analytical Chemistry, 50, 1290.
Wang, C. C., Juang, L. C., Lee, C. K., Hsu, T. C., Lee, J. F., & Chao, H. P. (2004). Effects of exchanged surfactant cations on the pore structure and adsorption characteristics of montmorillonite. Journal of Colloid and Interface Science, 280, 27–35.
Wang, T., Li, C., Wang, C. Q., & Wang, H. (2018). Biochar/MnAl-LDH composites for Cu(ΙΙ) removal from aqueous solution. Colloids and Surfaces, A: Physicochemical and Engineering Aspects, 538, 443–450.
Weber, W. J., & Morris, J. C. (1962). Advances in water pollution research: removal of biologically resistant pollutant from waste water by adsorption, in Proceedings of the International Conference on Water Pollution Symposium (Vol. 2, pp. 231–266). Oxford: Pergamon Press.
Wei, W., Bediako, J. K., Kim, S., & Yun, Y. S. (2016). Removal of cd(II) by poly(styrenesulfonic acid)-impregnated alginate capsule. Journal of the Taiwan Institute of Chemical Engineers, 61, 188–195.
Xie, Y. Y., Yuan, X. Z., Wu, Z. B., Zeng, G. M., Jiang, L. B., Peng, X., & Li, H. (2019). Adsorption behavior and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon spheres on the removal of Pb(II) and Cu(II). Journal of Colloid and Interface Science, 536, 440–455.
Ye, L. J., Chai, L. Y., Li, Q. Z., Yan, X., Wang, Q. W., & Liu, H. (2016). Chemical precipitation granular sludge (CPGS) formation for copper removal from wastewater. RSC Advances, 6, 114405–114411.
Yu, Y., & Chen, J. P. (2015). Key factors for optimum performance in phosphate removal from contaminated water by a Fe-Mg-La tri-metal composite sorbent. Journal of Colloid and Interface Science, 445, 303–311.
Zhao, D. L., Sheng, G. D., Hua, J., Chen, C. L., & Wang, X. K. (2011). The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chemical Engineering Journal, 171, 167–174.
Zhao, M. Q., Zhang, Q., Huang, J. Q., & Wei, F. (2012). Hierarchical nano-composites derived from nano-carbons and Layered double hydroxides - properties, synthesis, and applications. Advanced Functional Materials, 22, 675–694.
Zhou, H. G., Jiang, Z. M., & Wei, S. Q. (2013). A novel absorbent of nano-Fe loaded biomass char and its enhanced adsorption capacity for phosphate in water. Journal of Chemistry, 4, 1–9.
Zhou, H. G., Jiang, Z. M., & Wei, S. Q. (2018a). A novel hydrotalcite-like absorbent FeMnMg-LDH and its adsorption capacity for Pb2+ ions in water. Applied Clay Science, 153, 29–37.
Zhou, H. G., Jiang, Z. M., Wei, S. Q., & Liang, J. (2018b). Adsorption of Cd(II) from aqueous solutions by a novel layered double hydroxide FeMnMg-LDH. Water, Air, and Soil Pollution, 229, 78.
Zhu, X. H., Li, J., Luo, J. H., Jin, Y., & Zheng, D. (2017). Removal of cadmium(II) from aqueous solution by a new adsorbent of fluor-hydroxyapatite composites. Journal of the Taiwan Institute of Chemical Engineers, 70, 200–208.
Zhu, S. D., Khan, M. A., Wang, F. Y., Bano, Z., & Xia, M. Z. (2019). Rapid removal of toxic metals Cu2+ and Pb2+ by amino trimethylene phosphonic acid intercalated layered double hydroxide: a combined experimental and DFT study. Chemical Engineering Journal, 123711.
Funding
This research is supported by and China Postdoctoral Science Foundation funded project (Grant No. 2018M643421) and the Chongqing Postdoctoral Science Special Foundation under Grant No. XmT2018027.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 1097 kb)
Rights and permissions
About this article
Cite this article
Zhou, H., Tan, Y., Gao, W. et al. Removal of Copper Ions from Aqueous Solution by a Hydrotalcite-Like Absorbent FeMnMg-LDH. Water Air Soil Pollut 231, 370 (2020). https://doi.org/10.1007/s11270-020-04714-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04714-8