Abstract
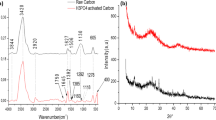
Activated carbon was derived from waste wood pallets in Hong Kong via phosphoric acid activation and applied to adsorption of basic dye (methylene blue), acid dyes (acid blue 25 and acid red 151), and reactive dye (reactive red 23). The results showed that respective adjustment in phosphoric acid concentration, impregnation ratio, activation temperature, and activation time could maximize the surface area and pore volume of activated carbon. An increase of impregnation ratio or activation temperature significantly influenced the pore size distribution by expanding the porous structure and creating more macropores than micropores. The characterization of the carbon surface chemistry using Fourier-transform infrared (FTIR) spectroscopy, however, revealed a decrease in the amount of several functional groups with increasing activation temperature. The physical properties (surface area and pore volume) of the wood waste-derived activated carbon (using 36% phosphoric acid with an impregnation ratio of 1.5 at an activation temperature of 550°C for 1.5 h) were comparable to those of commercial activated carbon (Calgon F400). The contrasting pH effects on the adsorption of different classes of dyes signified the importance of both electrostatic interaction and chemical adsorption, which correlated to pH-dependent dissociation of surface functional groups. It is noteworthy that the physical properties of activated carbon were insufficient to account for the observed dye adsorption behavior, whereas the surface chemistry of activated carbon and the nature and chemical structure of dyes were more important. The fast kinetics and high capacity of dye adsorption of wood waste-derived activated carbon suggest that production of activated carbon from different types of wood waste should merit further investigation.






Similar content being viewed by others
References
Ahmedna, M., Marshall, W. E., & Rao, R. M. (2000). Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties. Bioresource Technology, 71, 113–123.
Ahn, S., Werner, D., Karapanagioti, H. K., McGlothlin, D. R., Zare, R. N., & Luthy, R. G. (2005). Phenanthrene and pyrene sorption and intraparticle diffusion in polyoxymethylene, coke, and activated carbon. Environmental Science & Technology, 39, 6516–6526.
Allen, S. J. (1996). Types of adsorbent materials. In G. McKay (Ed.), Use of adsorbents for the removal of pollutants from wastewater. Boca Raton: CRC.
Banat, I. M., Nigam, P., Singh, D., & Marchant, R. (1996). Microbial decolorization of textile-dye-containing effluents: A review. Bioresource Technology, 58, 217–227.
Bellamy, L. J. (1954). The infra-red spectra of complex molecules. New York: Wiley.
Benjamin, M. M. (2002). Adsorption reactions. Water Chemistry, 550–627, McGraw-Hill.
Calgon Carbon Corporation (1999). Information bulletin: Activated carbon – What is it, how does it work. IB-1013–12/99.
Corbridge, D. E. C. (1956). Infra-red analysis of phosphorous compounds. Journal of Applied Chemistry, 6, 456–465.
Crittenden, J. C., Luft, P., Hand, D. W., Oravitz, J. L., Loper, S. W., & Arl, M. (1985). Prediction of multicomponent adsorption equilibria using ideal adsorbed solution theory. Environmental Science & Technology, 19, 1037–1043.
Daifullah, A. A. M., & Girgis, B. S. (1998). Removal of some substituted phenols by activated carbon obtained from agricultural waste. Water Research, 32, 1169–1177.
Derbyshire, F., Jagtoyen, M., Andrews, R., Rao, A., Martin-Gullon, I., & Grulke, E. (2001). Carbon materials in environmental applications. In L. R. Radovic (Ed.), Chemistry and physics of carbon, 27 (pp 1–66). New York: Marcel Dekker.
Diao, Y., Walawender, W. P., & Fan, L. T. (2002). Activated carbons prepared from phosphoric acid activation of grain sorghum. Bioresource Technology, 81, 45–52.
Diaz-Diez, M. A., Gomez-Serrano, V., Gonzalez, C. F., Cuerda-Correa, E. M., & Macias-Garcia, A. (2004). Porous texture of activated carbons prepared by phosphoric acid activation of woods. Applied Surface Science, 238, 309–313.
Dogan, M., Alkan, M., Turkylmaz, A., & Ozdemir, Y. (2004). Kinetics and mechanism of removal of methylene blue by adsorption onto perlite. Journal of Hazardous Materials, 109, 141–148.
Environment, Transport and Works Bureau (ETWB) (2003). Environment Hong Kong 2003 – Waste – Resource Materials and Decision Time, HKSAR, China.
Faria, P. C. C., Orfao, J. J. M., & Pereira, M. F. R. (2004). Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Research, 38, 2043–2052.
Frank, P. V. D. Z. (2002). Anaerobic azo dye reduction. PhD Dissertation, Wagenlingen University, Wageningen, Netherlands.
Gergova, K., & Eser, S. (1996). Effects of activation method on the pore structure of activated carbons from apricot stones. Carbon, 34, 879–888.
Ho, Y. S., Ng, J. C. Y., & McKay, G. (2000). Kinetics of pollutant sorption by biosorbents: Review. Separation and Purification Methods, 29, 189–232.
Hong Kong Environmental Protection Department (HKEPD) (2005). Monitoring of solid waste in Hong Kong. Waste Statistics for 2005, HKSAR, China.
Huang, L., Boving, T. B., & Xing, B. (2006). Sorption of PAHs by aspen wood fibers as affected by chemical alterations. Environmental Science & Technology, 40, 3279–3284.
Jain, A. K., Gupta, V. K., Bhatnagar, A., & Suhas, S. (2003). Utilization of industrial waste products as adsorbents for the removal of dyes. Journal of Hazardous Materials, B101, 31–42.
Juang, R. S., Wu, F. C., & Tseng R. L. (2000). Mechanism of adsorption of dyes and phenols from water using activated carbons prepared from plum kernels. Journal of Colloid and Interface Science, 277, 437–444.
Kacha, S., Derriche, Z., & Elmaleh, S. (2003). Equilibrium and kinetics of color removal from dye solutions with bentonite and polyaluminum hydroxide. Water Environment Research, 75, 15–20.
Kennedy, J., Mohan, das. K., & Sekaran, G. (2004). Integrated biological and catalytic oxidation of organics/inorganics in tannery wastewater by rice husk based mesoporous activated carbon––Bacillus sp. Carbon, 42, 2399–2407.
Khraisheh, M. A. M., Al-degs, Y. S., Allen, S. J., & Ahmad, M. N. (2002). Elucidation of controlling steps of reactive dye adsorption on activated carbon. Industrial & Engineering Chemistry Research, 41, 1651–1657.
Li, Q., Snoeyink, V. L., Marinas, B. J., & Campos, C. (2003). Pore blockage effect of NOM on atrazine adsorption kinetics of PAC: The roles of PAC pore size distribution and NOM molecular weight. Water Research, 37, 4863–4872.
Macias-Garcia, A., Diaz-Diez, M. A., Gomez-Serrano, V., & Fernandez-Gonzalez, M. C. (2003). Technical note preparation and characterization of activated carbons made up from different woods by chemical activation with H3PO4. Smart Materials and Structures, 12, 24–28.
Marsh, H. (2001). Activated carbon compendium: A collection of papers from the journal carbon 1996–2000. Amsterdam: Elsevier.
Millward, R. N., Bridges, T. S., Ghosh, U., Zimmerman, J. R., & Luthy, R. G. (2005). Addition of activated carbon to sediments to reduce PCB bioaccumulation by polycheate (Neanthes arenaceodentata) and an amphipod (Leptocheirus plumulosus). Environmental Science & Technology, 39, 2880–2887.
Moreno-Castilla, C. (2004). Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon, 42, 83–93.
Nagano, H., Tamon, T., Adzumi, K. N., & Suzuki, T. (2000). Activated carbon from municipal waste. Carbon, 38, 915–920.
Nakagawa, K., Namba, A., Mukai, S. R., Tamon, H., Ariyadejwanich, P., & Tanthapanichakoon, W. (2004). Activated carbon form municipal waste. Water Research, 38, 1791–1798.
O’Neill, C., Hawkes, F. R., Hawkes, D. L., Lourenco, N. D., Pinheiro, H. M., & Delee, W. (1999). Colour in textile effluents – Sources, measurement, discharge consents and simulation: A review. Journal of Chemical Technology and Biotechnology, 74, 1009–1018.
Monteiro, Jr., O. A. C., & Airoldi C. (1999). Some thermodynamic data on copper–chitin and copper–chitosan biopolymer interactions. Journal of Colloid and Interface Science, 212, 212–219.
Pelekani, C., & Snoeyink, V. L. (1999). Competitive adsorption in natural water: Role of activated carbon pore size. Water Research, 33, 1209–1219.
Pelekani, C., & Snoeyink, V. L. (2001). A kinetic and equilibrium study of competitive adsorption between atrazine and Congo red dye on activated carbon: The importance of pore size distribution. Carbon, 39, 25–37.
Pereira, M. F. R., Soares, S. F., Orfao, J. J. M., & Figueiredo, J. L. (2003). Adsorption of dyes on activated carbons: Influence of surface chemical groups. Carbon, 41, 811–821.
Philip, C. A., & Girgis, B. S. (1996). Adsorption characteristics of microporous carbons from apricot stones activated by phosphoric acid. Journal of Chemical Technology and Biotechnology, 67, 248–254.
Randtke, S. J., & Snoeyink, V. L. (1983). Evaluating GAC adsorptive capacity. Journal of the American Water Works Association, 75, 406–413.
Robinson, T., McMullan, G., Marchant, R., & Nigam, P. (2001). Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77, 247–255.
Rozada, M., Otero, J. B., Parra, A. M., & García, A. I. (2005). Producing adsorbents from sewage sludge and discarded tyres: Characterization and utilization for the removal of pollutants from water. Chemical Engineering Journal, 114, 161–169.
Senthilkumaar, S., Kalaamani, P., Porkodi, K., Varadarajan, P. R., & Subburaam, C. V. (2006). Adsorption of dissolved Reactive red dye from aqueous phase onto activated carbon prepared from agricultural waste. Bioresource Technology, 97, 1618–1625.
Socrates, G. (1994). Infrared characteristic group frequencies. New York: Wiley.
Sparks, D. L. (1999). Soil physical chemistry (2nd ed.). New York: CRC Press.
Sparks, D. L. (2003). Environmental soil chemistry (2nd ed.). Amsterdam: Academic.
Tanthapanichakoon, W., Ariyadejwanich, P., Jathong, P., Nakagawa, K., Mukai, S. R., & Tamon, H. (2005). Adsorption–desorption characteristics of phenol and reactive dyes from aqueous solution on mesoporous activated carbon prepared from waste tires. Water Research, 39, 1347–1353.
Toles, C. A., Marshall, W. E., & Johns, M. M. (1998). Phosphoric acid activation of nutshells for metals and organic remediation: Process optimization. Journal of Chemical Technology and Biotechnology, 72, 255–263.
Toles, C. A., Marshall, W. E., & Johns, M. M. (1999). Surface functional groups on acid-activated nutshell carbons. Carbon, 37, 1207–1214.
Vandevivere, P. C., bianchi, R., Verstraete, W. (1998). Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. Journal of Chemical Technology and Biotechnology, 72, 289–302.
Weber, W. J., Jr., & Smith, E. H. (1987). Simulation and design models for adsorption processes. Environmental Science & Technology, 21, 1040–1050.
Werner, D., Higgins, C. P., & Luthy, R. G. (2005). The sequestration of PCBs in Lake Hartwell sediment with activated carbon. Water Research, 39, 2105–2113.
Wong, Y. C., Szeto, Y. S., Cheung, W. H., & McKay, G. (2003). Equilibrium studies for acid dye adsorption onto chitosan. Langmuir, 19, 7888–7894.
Zhang, F. S., Nriagu, J. O., & Itoh, H. (2005). Mercury removal from water using activated carbons derived from organic sewage sludge. Water Research, 39, 389–395.
Zimmerman, J. R., Ghosh, U., Millward, R. N., Bridges, T. S., & Luthy, R. G. (2004). Addition of carbon sorbents to reduce PCB and PAH bioavailability in marine sediments: Physicochemical tests. Bioresource Technology, 38, 5458–5464.
Zimmerman, J. R., Werner, D., Ghosh, U., Millward, R. N., Bridges, T. S., & Luthy, R. G. (2005). Effects of dose and particle size on activated carbon treatment to sequester polychlorinated biphenyls and polycyclic aromatic hydrocarbons in marine sediments. Environmental Toxicology and Chemistry, 24, 1594–1601.
Acknowledgements
The authors gratefully acknowledge the financial support of the University Grants Council for Emerging High Impact Area (project no. HIA 04/04.EG03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsang, D.C.W., Hu, J., Liu, M.Y. et al. Activated Carbon Produced from Waste Wood Pallets: Adsorption of Three Classes of Dyes. Water Air Soil Pollut 184, 141–155 (2007). https://doi.org/10.1007/s11270-007-9404-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9404-2