Abstract
Chemically activated carbons synthesized from pine sawdust were applied efficiently for the elimination of wood dyes from aqueous solutions. Different proportions (1:2 and 1:4) of activating agent (KOH) and activation temperatures (600 and 850 °C) were used. Carbon surface morphology was characterized. The effect of pH (2–12), initial adsorbate concentration (5–500 mg L−1), and carbon dosage (0.1–0.5 g L−1) on dye adsorption were studied in batch mode. Langmuir model described well the adsorption equilibrium. The maximum found adsorption capacities were 1221.58, 1673.03, and 240.38 mg g−1 for blue and red at 500 mg L−1 and black at 100 mg L−1, respectively, using activated carbon at 850 °C and 1:4 (ACPS-4–850); at 25 °C, adsorbent dose 0.4 g L−1 for blue and black and 0.3 g L−1 for red dye and without change the pH for blue and red and at pH = 2 for black dye. The pseudo-second-order model explained the kinetics of adsorption except for the black dye at 100 mg L−1 using ACPS-4–850 for which it was the pseudo-first-order model. Desorption studies performed with ACPS-4–850 revealed that the adsorption was irreversible by chemical regeneration, whereas for the black dye, regeneration was efficient using H2O2 as desorbing agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A huge concern in the past years is the contamination of the aquatic environment, with the depletion of freshwater resources. Water quality can be affected by human activities including agriculture, mining, and the generation of energy. Various industries such as textile, food, wood, and paper are a significant source of contamination since typically include the use of harmful and toxic substances, including sulfur dioxide, nitrogen oxide, heavy metals or dyes. Particularly, dyes can contribute significantly to water pollution due to the use of great amounts in industries to color their products that are subsequently released into the water system [1,2,3]. Thus, if they are not treated properly, will dramatically affect the ecosystem at long term because dyes are not easily biodegradable due to their stable and complex chemical structure [4]. It is possible to classify dyes in three groups: anionic (substantive, acidic, and reactive dyes), cationic (alkaline dyes), and non-ionic (granular dyes) [5]. It was estimated that there are more than 100,000 types of dyes available in the world, and the textile industry discharges around 280,000 tons of dyes into water streams each year [6]. These dyes are dangerous not only for the aquatic biota but also for the human health because some of them presented toxicity and carcinogenicity, demanded their treatment before discharged to the environment [7].
Numerous techniques, such as coagulation, flocculation, chemical oxidation, membrane process, electrochemical process, filtration, reverse osmosis, ion exchange, advance oxidation, evaporation, and magnetic separations, have been used for the elimination of organic pollutants from water system [5, 8]. Adsorption was revealed to be one of the most effective ones, due to its characteristics of easy operation, environment-friendly, cost-effective, low health risk, sensitivity towards noxious pollutants, and non-destructive process [9]. Interesting adsorbents are porous materials, which can be modified to a desired degree of porosity through the use of different activation methods, conditions, and raw materials [10]. Usually, activated carbon (AC) refers to a material rich in carbon and with a well-designed internal pore structure, being the most used adsorbent. AC is characterized by a high surface area, well organized porous structure, favorable thermal stability, mechanical strength, and a variability of chemical functional groups on the surface [8, 10, 11]. However, the manufacture of AC using non-renewable materials (like coal or lignite) is still expensive and requires intensive energy, whereby biomass waste materials have been investigated as raw resources to produce AC due to their abundance and lack of toxicity [8]. In particular, lignocellulosic biomass arise as a promising green precursor since it is renewable, ecologically suitable, and cost effective [10]. In literature, various biomass materials were investigated to produce AC for dye adsorption such as stones of Ziziphus nummularia, Intsia bijuga sawdust, rubber seed and its shell, and Cornulaca monacantha stem [4, 5, 7, 8]. Usually, the AC synthesis comprises two steps: (1) the carbonization of the precursor and (2) its physical (with CO2 or water vapor) or chemical (with KOH, H3PO4 or ZnCl2) activation [4]. Additionally, the activation can be carried out through direct chemical activation (physical mixing) or by impregnation in which the precursor carbonization step is often eliminated. Potassium hydroxide has been widely used to produce low-cost AC since it is eco-friendly, leads to an increased oxygen functionality on carbon surface, generates high surface area, tailored pore size distribution, low environmental pollution, and low corrosiveness. Moreover, previous studies demonstrated the effectiveness of KOH-AC for dye adsorption [12, 13].
The precursor’s characteristics and the preparation conditions strongly influence AC characteristics, being important a careful choice of the precursor considering the availability and price of the material [4]. Pinus radiata stands out as a quick growing species broadly planted today, mostly in the southern hemisphere, and with a wide range of applications. Pine wood is used by the pulp and paper industries as well as for furniture and construction generating a substantial amount of waste (wood chips, twigs, sawdust, etc.) [14, 15]. Particularly in Galicia (northwestern Spain), Pinus radiata is the most frequently employed conifer for reforestation and covers an area of around 90,000 ha [16]. Pine sawdust (PS) from Pinus radiata is a low cost and abundantly available residue often discarded as waste or burnt to produce bioenergy, so its re-use to produce AC can be very worthwhile both for waste treatment and pollution reduction. Studies on dyes adsorption by ACs derived from pine sawdust are limited [17,18,19,20], and there are no studies with wood dyes. As mentioned, due to the disposal of untreated industrial wastewater and the disadvantages of using commercial activated carbon which limit its applicability, the valorization of biomass wastes to produce activated carbons for the removal of organic pollutants is of extreme importance [21, 22]. Thus, the objective of this work is to produce one biochar and three ACs from pine sawdust biomass and assess their adsorption capacity to eliminate blue, red, and black acid wood dyes. For this purpose, a study of adsorption, by analyzing the effect of several significant parameters on dye adsorption such as contact time, initial adsorbate concentration, initial pH, and adsorbent dose, was carried out. After carbonization, pine sawdust was chemically activated using KOH to improve the surface area and porosity trying to enhance dye adsorption. The exceptional adsorption capacity of activated carbons is mainly due to the well-developed porous structure, pore size distribution, and the presence of distinct functional groups [23]. Thus, in the present research, the production of activated carbons from a wood waste is proposed in an attempt to find environmentally friendly substitutes for commercial activated carbons to remove wood dyes from wastewaters. The determination of point of zero charge (pHPZC), scanning electron microscopy together with energy-dispersive X-ray spectrometry (SEM–EDX), and FTIR analyses were employed to characterize the adsorbents. Kinetic and equilibrium studies were conducted in controlled settings to evaluate the adsorption mechanism.
2 Experiments and methods
2.1 Chemicals
Potassium hydroxide (KOH, 85%, Probus), hydrochloric acid (HCl, 37%, Sigma Aldrich), and hydrogen peroxide (H2O2, 33%, Quimipur) were used as received. Acid dyes: blue for wood AGN-270% (C22H16N3NaO6S), red for wood GRA-200% (C17H11F3N3NaO4S+), and black Hispalan M-RN-140% (C40H20CrN6NaO14 \({S}_{2}^{-2}\)) were supplied by a wood company in Galicia (Spain). Its chemical structure is shown in Fig. 1. Standard solutions were set at 500 mg L−1, and the solutions with lower concentrations were achieved by diluting the most concentrated solution using distilled water.
2.2 Preparation of biochar and activated carbons
Pine (Pinus radiata) sawdust was prepared as in Pimentel et al. [24] and applied as an organic precursor for the production of biochar (BC) and ACs.
Carbonization procedure was described in Pimentel et. al [24]. Thereafter, the BC was mixed with a mortar with KOH as the activating agent at the desired weight ratio 1:2 or 1:4 (w/w) and activated under N2 atmosphere (10 mL min−1) for 2 h at two different activating temperatures, 600 °C or 850 °C. The acquired material was rinsed with HCl 0.1 M under stirring for 15 min in a solid/liquid ratio of 1/100 (g/mL) and with distilled water until stable pH and, finally, dried overnight at 105 °C [25]. The ACs prepared were called ACPS-2–850 (1:2 w/w and 850 °C), ACPS-4–850 (1:4 w/w and 850 °C), and ACPS-4–600 (1:4 w/w and 600 °C).
2.3 Characterization of biochar and activated carbons
The point of zero charge (pHPZC) of the materials was determined through the method described by Pimentel et al. [24].
Scanning electron microscopy (SEM) coupled with energy dispersive X-ray analysis (EDX) (ZEISS EVO LS 15 microscope) was used to examine the morphology of BC and ACs before and after adsorption. Samples were placed covering the carbon tape placed on the surface of the pin stub. Furthermore, the carbons were evaluated by Fourier transform infrared spectroscopy (FTIR), using a VARIAN FTIR 670 spectrometer, before and after adsorption [26].
2.4 Batch adsorption experiments
Adsorption experiments in batch mode were performed in a hydro shaking water bath (H20 SOW-LAUDA) at stirring rate of 210 rpm and 25 °C. The experiments were conducted by varying the pH (around 2, 6, 7, 9, and 12), initial concentration of dye (5 and 500 mg L−1), and carbon dosage (between 0.5 and 0.1 g L−1) to analyze the elimination of blue, red, and black wood dyes using the BC and ACs prepared. The procedure was the same as performed by Pimentel et al. [24].
The percentage of dye adsorbed was employed for calculating the dye removal efficiency (Eq. (1)). The adsorption capacity of the adsorbent was determined based on the amount adsorbed of dye per mass (Eq. (2)).
where C0 and C are the initial and the residual dye concentration, respectively (mg L−1), q is the capacity of adsorption (mg g−1), V is the volume of the dye dissolution (L), and m is the dry mass of adsorbent used (g).
2.5 Adsorption kinetics
Pseudo-first-order, pseudo-second-order, and intraparticle diffusion models were employed to assess the kinetic data of dyes adsorption and establish the dynamics of the adsorption processes whichever they are physical or chemical.
The linear pseudo-first-order kinetic model, recognized as Lagergren equation, is as indicated in Eq. 3.
where k1 (min−1) is the first-order-reaction rate constant, q and qe (mg g−1) represent the adsorption capacity at time t and equilibrium time, respectively.
One linear form of pseudo-second-order equation is expressed as (Eq. (4)):
where k2 (g mg−1 min−1) is the constant rate of the pseudo-second-order model [4, 27].
Intraparticle diffusion equation (Weber-Morris model) is given by Eq. (5).
where ki is the intraparticle diffusion rate constant (mg g−1 min−0.5), and C is the kinetic constant that gives information about the boundary layer thickness [4, 28].
The values of the model’s parameters were evaluated from the slope and y-intercept for the three linear equations (Eqs. 3–5). The appropriateness of the models to fit the experimental data was calculated from the determination coefficient (R2).
2.6 Adsorption equilibrium
The study of the adsorption equilibrium was performed considering the best operational conditions chosen according to the results of the previous experiments (Table 1).
For equilibrium experiments, the corresponding amount of each AC according to the solid–liquid ratio was added individually to solutions with distinct dye concentrations in Erlenmeyer flasks. The mixes were stirred at 210 rpm and 25 °C in a shaking water bath (H20 SOW-LAWDA) for the selected time. To depict adsorption process, the linearized forms of Langmuir (Eq. (6)), Freundlich (Eq. (7)), Temkin (Eq. (8)), and Dubinin-Radushkevich (Eq. (9)) models were used.
where Ce is the concentration of dye at equilibrium (mg L−1); qe is the adsorption capacity at equilibrium (mg g−1); qmax and qm,D are the maximum adsorption capacities (mg g−1); KL corresponds to the Langmuir adsorption constant and denotes the bonding energy (L mg−1); KF and n are Freundlich model constants; B is equal to (RT)/bT and is associated to the heat of adsorption (J mol−1); bT (kJ mol−1) and KT (L g−1) are Temkin model constants, R is universal constant of gases (J mol−1 K−1), T (K) is absolute temperature, ɛ (kJ mol−1) is Polanyi coefficient and is equal to RTln(1 + 1/Ce), KDR is activity coefficient (mol2 J−2) and is associated to the adsorption energy. In the Langmuir model, RL is an important characteristic and is equal to 1/(1 + KLC0), where C0 is the initial dye concentration [29,30,31,32].
2.7 Desorption and regeneration
In an attempt to regenerate the AC with the best results for dyes adsorption (ACPS-4–850), H2O2 was used as a desorbing agent evaluating the removal efficiency (Eq. (1)) in consecutive cycles. The dye-saturated AC was put in contact with 0.3% w/v H2O2 solution and were left in agitation at 330 rpm for 4 h (Cienytech, VWR) at 25 °C. Then, the adsorbent was washed with distilled water and dried at 50 °C [33, 34]. The adsorbent was put again in contact with dye solution as mentioned before.
Also, to investigate the possibility of the reusability of ACPS-4–850 by thermal regeneration, the saturated material with blue and red dyes was carbonized at 350 °C for 1.5 h in a pure N2 (10 mL min−1) atmosphere at 5 °C min−1 heating rate, and finally cooled with N2 flow [35].
2.8 Statistical analysis
For all studies that present statistical analysis, the presence of significant differences among the dyes adsorption efficiency results shown for ACPS was evaluated according to Pimentel et al. [24].
3 Results and discussion
3.1 Characterization of BC and ACs
The pHPZC stands for the pH level at which the charge on the adsorbent surface is zero, meaning that the electrostatic interactions between the material’s positive and negative charges are equal. Adsorbent surface becomes positive (existence of H+) or negative (presence of OH−) charged when the pH is lower and higher than pHPZC, respectively. At pH < pHPZC, the surface is positively charged and could favor the electrostatic attraction of the anionic dyes used in the present work [4, 36]. For BC, ACPS-4–850, ACPS-4–600, and ACPS-2–850, pHPZC was found to be 6.7, 7.2, 4.0, and 7.1, respectively (Fig. 2).
SEM was utilized to analyze the micro-morphological structure and surface texture of the BC and ACs. SEM micrographs of the materials are presented in Fig. 3(a–d).
SEM images of produced materials. a BC, b ACPS-4–850, c ACPS-4–600, and d ACPS-2–850 at 5000 × magnification before adsorption and after adsorption with blue dye for ACPS-4–850 (e), ACPS-4–600 (f), and ACPS-2–850 (g), with red dye for ACPS-4–850 (h), ACPS-4–600 (i), and ACPS-2–850 (j), and with black dye for ACPS-4–850 (k), ACPS-4–600 (l), and ACPS-2–850 m at 5000 × magnification
All carbons showed the same microstructure. An array of uniform porous openings with circular shape distributed around the surface is observed. BC presented a roughness surface, and it is possible to observe that preserved the cell wall structure of the lignocellulosic materials while the ACs presented a flat surface, except for ACPS-2–850 (d) possibly due to the lower quantity of KOH used for the activation, which resulted in a less aggressive action [37, 38].
Surface morphology of the materials facilitated dye adsorption. As shown in Fig. 3(e–m), pores were filled with the dye solution which can cause saturation of the adsorbent binding sites [39].
Surface elemental composition of all the samples determined by EDX analysis before and after adsorption are presented in Table 2. Regarding BC, the elemental analysis was not performed given the poor dye adsorption [24]. The EDX analysis revealed the existence of elements from the molecular structure of dyes on the surface of ACs (Fig. 1) which confirmed the capability of AC to adsorb anionic dyes.
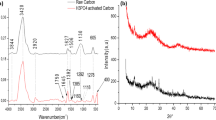
FTIR spectra of the materials in the range 400–4000 cm−1 were recorded to better understand which functional groups at the surface are responsible for dye adsorption (Fig. 4 and Table 3).
Some adsorption bands disappeared after activation process (Fig. 4a, b, c, d), indicating that organic matter vaporization occurred and lead to pore formation [40]. High intensity vibration can be observed at 3400 cm−1, which is due to –OH tensile vibrations. Also, modifications to the functional groups happened after chemical activation, most notably with the decrease of the strength of the bands of 3400, 2919, 2852, and 1575 cm−1. After dye adsorption, several peaks’ shapes and intensities changed, because of the interaction and positioning of the dyes on the adsorbent surface. For instance, the range of –OH vibrations changed for ACPS-4–850 from 3461 to 3476 cm−1 for blue and red and to 3444 cm−1 for black, for ACPS-4–600 from 3421 to 3401 cm−1 for blue and red and to 3432 cm−1 for black, and for ACPS-2–850 from 3463 to 3450 cm−1 for blue and black and to 3436 cm−1 for red, which suggests that throughout the adsorption process, hydrogen bonds have been established [29]. Only the ACPS-2–850, after the adsorption with black dye, showed a major peak at around 1400 cm−1 due to the skeletal C = C vibrations of aromatic rings.
Regarding BC, the characterization was performed in order to evaluate the efficacy and improvement achieved with the activation process. Adsorption experiments were not conducted since as seen in a previous study, the removal efficiency and adsorption capacity of the dyes used were too low, less than 26% and 3 mg g−1 at 5 mg L−1, respectively [24].
3.2 Batch adsorption experiments
3.2.1 Influence of pH and initial dye concentration
The pH of the solution changes the charge of adsorbent surface and the ionization degree of the adsorbate, and consequently, it can affect dye adsorption due to the existence of electrostatic interactions between dye and carbon surface. Though, hydrophobic interactions, π-π interactions, and hydrogen bonds can also control the adsorption process [7, 39]. Figure 5 illustrated the influence of pH on the adsorption efficiency of the ACs for the blue, red, and black dyes.
Influence of the pH and initial dye concentration on the adsorption of blue, red, and black dyes onto ACPS-4–850 (a, b), ACPS-4–600 (c, d), and ACPS-2–850 (e, f) (adsorbent dosage 0.5 g L−1, agitation rate 210 rpm, 25 °C, natural pH at 5 mg L−1: 6.0, 5.3, and 5.7 for blue, red, and black, respectively, at 25 mg L−1: 4.9 for black dye, at 100 mg L−1: 8.1 and 5.5 for red and black dyes respectively and at 500 mg L−1: 6.2, 9.3, and 5.4 for blue, red, and black respectively; contact time: 48 h for blue and red dyes and 12 h for black)
For 5 mg L−1 (Fig. 5 (a, c, e)), the higher removal efficiency was above 95% at natural pH for blue and red dyes for all materials. In the case of black dye, the best adsorption efficiency (> 98%) was obtained at pH 2 for all ACs. Because AC’s surface is positively charged at low pH (pH < pHPZC), the better adsorption happens due to the strong electrostatic interaction between the AC surface and the anionic dyes. Also, the acid dissociation constant (pKa) of blue, red, and black dyes needs to be considered. Previous research [41] revealed that the pKa value of blue, red, and black dyes was 10.4, 10.6, and 10.3, respectively, which makes that dye molecules exist mostly as anionic salts in solution as they ionize and become negatively charged radicals [42]. As pH increases, the positive sites on the adsorbent surface diminish which impairs the electrostatic attraction, which was more evident in the case of ACPS-4–600 (Fig. 5 (c)) where the adsorption efficiency decreased. This fact agrees with the low pHPZC value for this material. The higher amount of adsorbed blue, red, and black dyes were 9.9, 14.4, and 10.3 mg g−1 on ACPS-4–850, 10.9, 10.1, and 9.5 mg g−1 on ACPS-4–600 and 8.5, 10.6, and 10.9 mg g−1 on ACPS-2–850. As in some cases, mainly for blue and red dyes, the influence of pH was low due to the great adsorption performance at all pH values, its effect increasing the initial concentration of dye (500, 100, and 25 mg L−1) was also investigated (see Fig. 5 (b), (d), and (f)).
A general decrease in dye uptake was seen with the increase in starting dye concentration at 500 mg L−1 (which is due to the excessive number of dye molecules relative to the amount of available adsorption sites), except for the ACPS-4–850 for the blue and red dyes and ACPS-2–850 for the blue dye [7]. Foroutan et al. [29] also reported a decrease in the adsorption efficiency at high dye concentrations. At lower concentrations, the dye molecules fill the available sites without competition and more pollutant can be adsorbed [39]. Also, the amount of dye adsorbed onto the ACs increased significantly with increasing concentration. The highest amount of adsorbed blue, red, and black dyes at 500 mg L−1 were 1010.9, 1068.7, and 492.2 mg g−1 on ACPS-4–850, 849.6, 363.7, and 69.9 mg g−1 on ACPS-4–600 and 1007.6, 837.5, and 519.3 mg g−1 on ACPS-2–850. Furthermore, the reduction in adsorption effectiveness at high dye concentrations can be due to the adsorbent active sites being saturated and the existence of repulsive electrostatic forces between the dye adsorbed and dye in solution [29]. It is evident that ACPS-4–850 showed the best adsorption performance followed by ACPS-2–850 and ACPS-4–600 which can be explained since the ACPS-4–850 had the highest total pore volume with the lowest percentage of microporosity (67.8%) combined with the largest internal surface area of 2864.5 m2 g−1 [43]. A smaller internal surface area of 2270.5 m2 g−1 was found for ACPS-2–850 and of 2437.2 m2 g−1 for ACPS-4–600 together with a smaller pore size [43] which means that a certain size exclusion can exist, and also, the influence of the dye molecules already adsorbed upon the diffusion process due to the proximity of the walls in the micropores.
To improve the adsorption process, when the removal percentage was lower than 80%, the initial dye concentration was reduced to 100 or 25 mg L−1. With ACPS-4–850 for the black dye reducing initial concentration to 100 mg L−1, adsorption removal percentage increased to 100%. With ACPS-4–600 and ACPS-2–850 reducing concentration to 100 mg L−1 and 25 mg L−1 for red and black dyes respectively, the removal percentage increased to 100% for red dye and 77.5% and 84.4%, respectively, for black dye. These results evidence the significant impact of initial dye concentration on the adsorption performance. The higher adsorption capacity of ACs in comparison to that of BC, reported in a previous study [24], can be due to the presence of porous structure which may result in a higher total surface area which contributes with more functional groups. These findings prove the efficiency of the chemical activation of the biomass to produce ACs.
Based on the experimental results obtained, pH 2 for black dye and red dye with ACPS850-K-2 or natural pH for blue and red dyes were selected for further investigations. A decrease in the pH needed to be performed when the adsorption was limited, mainly in the carbons with high microporosity. Baloo et al. [39] also stated that for the removal of anionic dyes by palm waste-derived ACs, the best adsorption results were obtained at low pH values. According to Malik [44], sawdust-derived AC was most effective in removing anionic direct dyes at pH 3 or lower.
3.2.2 Effect of adsorbent dose
Adsorbent dose can also affect the efficiency of the adsorption process and the adsorbent’s capacity, as well as the overall cost of the process mainly at large-scale application [10, 29]. The effect of adsorbent dose in dye removal efficiency and on the adsorption capacity is illustrated in Fig. 6.
At 5 mg L−1, the total removal of dyes was achieved in the entire range of adsorbent dose studied (0.5–0.1 g L−1) except for the black dye using ACPS-4–600 and ACPS-2–850 for which at 0.1 g L−1, the efficiency decreased from 99 to 78% and from 99 to 58%, respectively. The fact that part of the surface area of the adsorbents is not used in the dye adsorption process may allow the dose to be reduced, avoiding loss in the adsorbed quantity [10]. For blue and red dyes at 5 mg L−1, 0.1 g L−1 was the selected adsorbent dose with a removal efficiency of 100% and a maximum adsorption capacity of 53.8 mg g−1 and 44.0 mg g−1 for ACPS-4–850, 49.0 mg g−1 and 52.0 mg g−1 for ACPS-4–600, and 54.0 mg g−1 and 49.0 mg g−1 for ACPS-2–850, respectively. In the case of black dye 0.1 g L−1 was the dose chosen for ACPS-4–850, 0.2 g L−1 for ACPS-4–600, and 0.3 g L−1 for ACPS-2–850 with a maximum adsorption capacity of 45.2 mg g−1, 15.3 mg g−1, and 17.0 mg g−1 respectively. Regarding the initial dye concentration, the study was only performed if the efficiency was 100% at 0.5 g L−1. At higher concentrations, it could be noticed that in general, dye removal efficiencies decreased with decreasing the adsorbent dose. For ACPS-4–850, the selected dose was 0.4 g L−1 for the blue dye at 500 mg L−1 and for the black dye at 100 mg L−1, and 0.3 g L−1 for the red dye at 500 mg L−1, and the maximum adsorption capacity was 1411.0, 1635.0, and 232.1 mg g−1, respectively. Regarding ACPS-4–600, it was impossible to reduce the adsorbent dosage due to the low available surface caused by the high microporosity (89.0%) [43]. Concerning ACPS-2–850, it was only possible to decrease red dye’s adsorbent dose at 100 mg L−1, and 0.2 g L−1 was selected with a maximum adsorption capacity of 480.9 mg g−1. The adsorbent dose was selected considering that the adsorption process’ efficiency did not change significatively, which can be due to the decrease of dye concentration or the inexistence of contact between the dye and the adsorbent sites due to the collision between adsorbent particles and reduction of the active surface [29]. Generally, the ACs’ adsorption capacity rose with decreasing the adsorbent dose. This occurs since as the dose increases, the amount of surface (and mass of carbon) unavailable for size exclusion adsorption also increases, which leads to a reduction in adsorption capacity [29]. Previous studies [10, 29, 45] also reported that dye adsorption efficiency increases with increasing the adsorbent dose.
3.2.3 Adsorption kinetics
Adsorption kinetics was investigated to evaluate the adsorption mechanism and the rate limiting step. Experimental data were fitted to the linear pseudo-first-order (PFO), pseudo-second-order (PSO), and intraparticle diffusion models; and the predicted kinetic curves for the blue, red, and black dyes on ACPS-4–850, ACPS-4–600, and ACPS-2–850 are depicted in Fig. 7(a–f).
As observed, the adsorption of the red dye (the smaller one) is higher for ACPS-4–850 which presents the highest macro or mesoporosity and a 68% of microporosity [24]. Conversely, when ACs with high microporosity (ACPS-4–600, 89% and ACPS-2–850, 85%) are applied, it can be observed that the blue dye adsorption increases. With a high microporosity percentage, it is possible that the repulsion inside the pore walls or even with other molecules of dye adsorbed could affect the adsorption process [46].
At 5 mg L−1 (Fig. 7(a), (c), and (e)), the adsorption process occurred fast mainly for ACPS-4–850 that was less than 1 h for black dye and less than 5 min for blue and red dyes, and in a subsequent step, the rate steadily fell until it reached equilibrium because of fewer vacant sites and a decline in the concentration driving force. By varying the activation conditions, by decreasing the temperature or the ratio of activating agent, the equilibrium time for dye adsorption increased maybe due to diffusional limitations related to the small pore size that was more evident in the case of black dye. As shown in Fig. 7(b), (d), and (f), the adsorption capacity increased significantly with the rise in concentration. The highest adsorption capacity at equilibrium corresponded to ACPS-4–850, and it was 1221.58 mg g−1 for blue, 1673.03 mg g−1 for red, and 240.38 mg g−1 for black dye, and the equilibrium time was 4 h for the blue and red dyes and 9 h for the black dye. The calculated kinetic parameters are presented in Table 4.
Pseudo-second-order model satisfactorily fitted adsorption data, except for ACPS-4–850 with the black dye at 100 mg L−1 that was best fitted to the pseudo-first-order model (Table 4, Fig. 7) revealing the existence of physical adsorption. Although in some cases, the pseudo-first-order kinetic model gave high R2 values, calculated qe values are far from the experimental ones, revealing an inaccurate prediction. The appropriateness of the pseudo-second-order model demonstrated that chemical reactions between the AC and dyes can contribute to the adsorption process [39]. This suggests that the dyes were adsorbed onto the oxygenated active sites by surface exchange processes. In a second stage, the dye molecules diffused into the porous structure and were adsorbed within the inner pores of the ACs. The good fit (R2 > 0.9) to the intraparticle diffusion model for the first zone (Table 4) confirmed the proposed mechanism [47].
Thus, the intraparticle diffusion model was used to better understand the various adsorption process stages. When there is only one step and the linear model crosses the origin, it is believed that the only mechanism regulating the adsorption process is intraparticle diffusion. Regarding the experiments at high dye concentration, there are four cases where only one step of intraparticle diffusion occurred and with high R2 (> 0.9); however, the linear model did not cross the origin which implies that other adsorption mechanism is occurring. For the other cases, the adsorption process comprises two stages: the initial sharp step corresponds to the quick diffusion on the exterior surface, and the second phase is a progressive adsorption under the control of intraparticle diffusion [13]. Besides, it is possible to infer that the adsorption capacity was also influenced by the dye molecular size since the molecular size for blue (1.4 nm) and red (1.3 nm) was lower than that for black (1.5 nm), which is advantageous for the dye molecular diffusion in the adsorbent through the mesoporous filling mechanism [24, 48].
3.2.4 Equilibrium isotherms
Adsorption equilibrium data were fitted to the Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich isotherm models, and the results are shown in Fig. 8 and Table 5.
Adsorption isotherms of blue, red, and black dyes for ACPS-4–850 (a, b, c), ACPS-4–600 (d, e, f), and ACPS-2–850 (g, h, i) for selected conditions (Table 1)
For all ACs, dyes adsorption experimental data were best fitted to the Langmuir isotherm model (R2 > 0.9) which suggested that the adsorption occurs in monolayer on a surface with uniform energy sites similarly accessible for interaction. The values of Langmuir isotherm’s dimensionless separation factor (RL) varied from 0 to 1 for all concentrations demonstrating that the adsorption process is favorable [29]. This conclusion is supported by the values of n obtained using the Freundlich isotherm model that showed a favorable adsorption process since in all cases is greater than the unity [49]. Moreover, the average energy value (E) was calculated using D-R model and showed that the adsorption process is not physical because in any case, it was lower than 8 kJ mol−1. For the black dye, E is between 8 and 16 kJ mol−1 for all carbons which is related with ion exchange interactions and for the other two dyes and for every ACs is higher than 16 kJ mol−1 which implies that during the adsorption process, the molecules of dye undergo through diffusion [50, 51]. ACPS-4–850 showed higher adsorption capacity for the three dyes than the other carbons which can be explained by its larger surface area and higher mesoporosity [43]. Moreover, in the case of blue and red dyes, the isotherms can be categorized as type H2, according to the Giles classification, which indicates elevated affinity between the AC and the dye, as illustrated by the vertical behavior at low initial concentrations. The interaction between the dye and the carbons gets progressively more difficult, as concentration increases, which results in a concave curvature of the isotherms [49, 52]. On the other hand, the isotherm for the black dye due to the initial curvature presented with the increase on the concentration, can be classified as L2 which is particular for a strong intermolecular interaction [52].
In general, the adsorption capacity of the adsorbents changes with the carbons’ precursor, the adsorption process specifications, and with the class of pollutant [4]. So, numerous studies have been conducted to find suitable adsorbents with appropriate characteristics and great adsorption capacity. Table 6 shows the results for ACPS-4–850 with the maximum adsorption capacity at 500 mg L−1 for blue and red dyes and at 100 mg L−1 for black dye compared with other ACs derived from various biomass precursors used in similar dyes adsorption processes.
3.2.5 Desorption and regeneration
Reusing the adsorbent for economic profit is a crucial approach in an advanced adsorption process. The ability of AC to regenerate after numerous cycles of adsorption and desorption determines how cost-effectively dyes can be removed from aqueous solutions using AC. Thus, the recycle of the AC that led to the best results for dyes adsorption, ACPS-4–850, was investigated. Different solvents, namely NaOH (0.1 M; 10 M), HCl (0.1 M), ethanol 96%, acetone:water (3:2 v/v), acetone (> 99.5%), acetone 10%, methanol 10%, and NaCl 0.1 M were used for the desorption of dyes from the AC. Table 7 lists the percentage of desorption of blue, red, and black dyes by using these desorption agents.
The results indicate that the amount of dye desorbed in all cases and for the different dyes and conditions was rather low, which suggests that anionic dyes adsorption on ACPS-4–850 is generally irreversible by chemical regeneration. This conclusion is supported by the values of the separation factor (RL) calculated through the Langmuir model since they are very close to zero (Table 6) indicating that the adsorption system is irreversible. The same outcomes were reported by Tran et. al [60] for the desorption of cationic dyes from commercial activated-charcoal.
In a further attempt to regenerate the AC, hydrogen peroxide was tried as desorption agent [33, 61], and the removal efficiency in consecutive cycles are presented in Fig. 9.
As demonstrated by Fig. 9, the adsorption efficiencies of blue and red dyes significantly decreased after the second cycle of adsorption from 99.89 and 98.60 to 16.17% and 23.7%, respectively. This demonstrates that for these dyes, the H2O2 is also inefficient for the AC regeneration. On the contrary, for the black dye, the adsorption efficiency was higher than 80% until the third adsorption cycle and was reduced to 47.36% after five adsorption cycles. This can be due to the degradation of the AC when used multiple times or due to the saturation of the material surface [40]. For this dye, H2O2 considerably increased the effectiveness of the desorption process contrasted to previously essayed solvents (Table 7). This improvement of the efficiency can be attributed to a number of simultaneous mechanisms that are primarily facilitated by the interaction between the oxidizing agent and the AC surface. Nevertheless, for the degradation of highly toxic compounds, although H2O2 displays a comparatively significant redox potential (E0 = 1.77 V), chemical oxidation may not be effective, because it needs a high reaction energy to be activated in order to produce oxidizing species and break the O–O bond (bond energy is 213.8 kJ mol−1) [34]. Also, AC catalyzes the decomposition of H2O2 to produce oxidant species •OH the adsorbed dye [33, 34]. Usually, the desorption process produces a visible increase on the color intensity of the solution, which was not possible to observe. Possibly, the electrostatic interactions between the black dye and AC structure are strong, and the consequent release of dye from the active sites did not occur.
Finally, the carbon with adsorbed blue and red dyes underwent a thermal regeneration as proposed by Ma [35]. For the blue dye, the removal efficiency decreased from 99.89 to 28.72% and for red dye from 98.60 to 53.99% which revealed the inefficiency of thermal regeneration, most likely as a result of the thermal treatment-induced collapse of the carbon porous structure. So, for blue and red dyes, further studies should be performed to improve the regeneration of the ACPS-4–850.
3.2.6 Adsorption mechanism
Various types of interaction can occur between ACPS and dye molecules, as observed by the kinetic and equilibrium studies, and the suggested mechanism is given in Fig. 10. FTIR before adsorption confirmed the presence of functional groups which could effectively interact with the dyes molecules. In addition, SEM analysis showed well developed and distributed pores on the carbons leading to a large surface area that improves the adsorption process.
Moreover, based on pH influence, electrostatic interactions could happen between the anionic dyes and positive charged positions (carbonyl and oxygen-containing functional groups) that are present on the surface of the AC, particularly in neutral and acidic solutions. As seen in the FTIR result following dye uptake, another interaction that can take place between nitrogen in dyes structure and free H of oxygen-containing functional groups on the AC surface is hydrogen bonding. Finally, π-π interaction arises from interaction between dye aromatic rings and the hexagonal skeleton of ACs, as the electron-donating functional groups (–OH) of AC may increase π-donating strength of the aromatic ring [10].
4 Conclusions
High-quality activated carbons were prepared from pine sawdust biomass for the elimination of blue, red, and black wood dyes from wastewater. SEM results showed a uniform porous surface in all carbons. FTIR analysis demonstrated the presence of functional groups (hydroxyl, phenols, alcohols, alkane, and carboxyl) in the activated carbons available for dyes adsorption. The carbon activated with KOH 1:4 (w/w) at 850 °C for 2 h led to around 100% of dye removal efficiency and showed the best adsorption capacities of 1221.6, 1673.0, and 240.4 for blue, red, and black dyes, respectively, at 500 mg L−1 for the blue and red dyes and 100 mg L−1 for the black dye. The activated carbons with high SBET showed a qmax of 15.3 and 1673.0 mg g−1, respectively. The factors that affected the removal of dyes included pH, initial dye concentration, and ACPS dose. Dye removal increased by increasing ACPS dose (up to 0.5 g L−1) but decreased with increasing the initial dye concentration (up to 500 mg L−1), except for ACPS-4–850 for blue and red dyes that reached a 100% removal for any ACPS dose (0.1–0.5 g L−1) at the highest concentration essayed. Generally, the adsorption kinetics were best represented by the pseudo-second-order kinetic model, which showed that chemical adsorption was an important factor in the adsorption process. For the three dyes, equilibrium data were best fitted to the Langmuir model also representative of a monolayer chemical adsorption on the carbon surface. The reusability study showed that ACPS-4–850 can be re-utilized at least up to five cycles for black dye removal using hydroxide peroxide as desorbing agent with adsorption efficiencies higher than 80% until the third adsorption cycle but the desorption process was inefficient for the blue and red dyes.
Therefore, results showed that pine sawdust-based activated carbon is a novel and capable adsorbent for dyes removal. The results thus point to a promising adsorbent for the removal of wood dyes from water systems that is also safe for the environment. Future research should be conducted on the improvement of dyes desorption from the activated carbon, to reuse the adsorbent and make the process even more sustainable.
Data availability
All data generated or analyzed during this study are included in this article.
References
Streit AFM, Côrtes LN, Druzian SP et al (2019) Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Sci Total Environ 660:277–287. https://doi.org/10.1016/j.scitotenv.2019.01.027
Hu Y, Zou C, Xiong T, Wang H (2023) Strong adsorption of cationic dyes by cucurbit[7]uril modified magnetic carbon nanotubes: investigation on adsorption performance, reusability, and adsorption mechanism. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2023.07.062
Gholami A, Mousavi SB, Heris SZ, Mohammadpourfard M (2023) Highly efficient treatment of petrochemical spent caustic effluent via electro-Fenton process for COD and TOC removal: optimization and experimental. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-03772-2
Boudechiche N, Fares M, Ouyahia S et al (2019) Comparative study on removal of two basic dyes in aqueous medium by adsorption using activated carbon from Ziziphus lotus stones. Microchem J 146:1010–1018. https://doi.org/10.1016/j.microc.2019.02.010
Khasri A, Ahmad MA (2018) Adsorption of basic and reactive dyes from aqueous solution onto Intsia bijuga sawdust-based activated carbon: batch and column study. Environ Sci Pollut Res 25:31508–31519. https://doi.org/10.1007/s11356-018-3046-3
Xue H, Wang X, Xu Q et al (2022) Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: a comparative study by experimental and advanced modeling analysis. Chem Eng J 430:. https://doi.org/10.1016/j.cej.2021.132801
Nizam NUM, Hanafiah MM, Mahmoudi E et al (2021) The removal of anionic and cationic dyes from an aqueous solution using biomass-based activated carbon. Sci Rep 11:1–17. https://doi.org/10.1038/s41598-021-88084-z
Sharma A, Siddiqui ZM, Dhar S et al (2019) Adsorptive removal of congo red dye (CR) from aqueous solution by Cornulaca monacantha stem and biomass-based activated carbon: isotherm, kinetics and thermodynamics. Sep Sci Technol 54:916–929. https://doi.org/10.1080/01496395.2018.1524908
Reza MS, Yun CS, Afroze S et al (2020) Preparation of activated carbon from biomass and its’ applications in water and gas purification, a review. Arab J Basic Appl Sci 27:208–238. https://doi.org/10.1080/25765299.2020.1766799
Jawad AH, Abdulhameed AS, Bahrudin NN et al (2021) Microporous activated carbon developed from KOH activated biomass waste: surface mechanistic study of methylene blue dye adsorption. Water Sci Technol 84:1858–1872. https://doi.org/10.2166/wst.2021.355
Danish M, Ahmad T (2018) A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew Sustain Energy Rev 87:1–21. https://doi.org/10.1016/j.rser.2018.02.003
Heidarinejad Z, Dehghani MH, Heidari M et al (2020) Methods for preparation and activation of activated carbon: a review. Environ Chem Lett 18:393–415. https://doi.org/10.1007/s10311-019-00955-0
Jahan K, Singh V, Mehrotra N et al (2021) Development of activated carbon from KOH activation of pre-carbonized chickpea peel residue and its performance for removal of synthetic dye from drinking water. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01938-4
Santos TM, Rigual V, Oliet M et al (2019) Two-step fractionation of Pinus radiata by autohydrolysis and organosolv delignification for enzymatic hydrolysis. J Chem Technol Biotechnol 94:3951–3959. https://doi.org/10.1002/jctb.6197
Brockerhoff EG, Gresham BA, Meurisse N et al (2023) Pining away and at home: global utilisation of Pinus radiata by native and non-native insects. NeoBiota 84:137–167. https://doi.org/10.3897/NEOBIOTA.84.95864
Crecente-Campo F, Marshall P, LeMay V, Diéguez-Aranda U (2009) A crown profile model for Pinus radiata D. Don in northwestern Spain. For Ecol Manage 257:2370–2379. https://doi.org/10.1016/j.foreco.2009.03.038
Yang Y, Cannon FS (2022) Biomass activated carbon derived from pine sawdust with steam bursting pretreatment; perfluorooctanoic acid and methylene blue adsorption. Bioresour Technol 344:126161. https://doi.org/10.1016/j.biortech.2021.126161
Akmil-Başar C, Önal Y, Kiliçer T, Eren D (2005) Adsorptions of high concentration malachite green by two activated carbons having different porous structures. J Hazard Mater 127:73–80. https://doi.org/10.1016/j.jhazmat.2005.06.025
Yakout SM, Hassan MR, El-Zaidy ME et al (2019) Kinetic study of methyl orange adsorption on activated carbon derived from pine (Pinus strobus) sawdust. BioResources 14:4560–4574. https://doi.org/10.15376/biores.14.2.4560-4574
Zhu G, Xing X, Wang J, Zhang X (2017) Effect of acid and hydrothermal treatments on the dye adsorption properties of biomass-derived activated carbon. J Mater Sci 52:7664–7676. https://doi.org/10.1007/s10853-017-1055-0
Ahmed TA, Abdulhameed AS, Ibrahim S et al (2023) High surface area mesoporous activated carbon produced from Iraqi reed via pyrolysis assisted H3PO4 activation: Box-Behnken design for surfactant removal. Diam Relat Mater 133:109756. https://doi.org/10.1016/j.diamond.2023.109756
Uddin MK, Abd Malek NN, Jawad AH, Sabar S (2023) Pyrolysis of rubber seed pericarp biomass treated with sulfuric acid for the adsorption of crystal violet and methylene green dyes: an optimized process. Int J Phytoremediation 25:393–402. https://doi.org/10.1080/15226514.2022.2086214
Harrache Z, Abbas M (2022) Elimination of a cationic dye in aqueous solution by adsorption on activated carbon: optimization of analytical parameters, modeling and thermodynamic study. J Eng Fiber Fabr 17:. https://doi.org/10.1177/15589250221134343
Pimentel CH, Freire MS, Gómez-Díaz D, González-Álvarez J (2023) Preparation of activated carbon from pine (Pinus radiata) sawdust by chemical activation with zinc chloride for wood dyes adsorption. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-04138-4
Eleri OE, Azuatalam KU, Minde MW et al (2020) Towards high-energy-density supercapacitors via less-defects activated carbon from sawdust. Electrochim Acta 362:137152. https://doi.org/10.1016/j.electacta.2020.137152
Domínguez-Ramos L, Prieto-Estalrich A, Malucelli G et al (2022) (2022) N- and S-doped carbons derived from polyacrylonitrile for gases separation. Sustain 14:3760. https://doi.org/10.3390/SU14073760
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59. https://doi.org/10.1061/JSEDAI.0000430
Foroutan R, Peighambardoust SJ, Peighambardoust SH et al (2021) Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: a kinetic, equilibrium and thermodynamic study. Molecules 26:1–19. https://doi.org/10.3390/molecules26082241
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich H (1907) Über die adsorption in lösungen. Zeitschrift für Phys Chemie 57:385–470
Chandarana H, Suganya S, Madhava AK (2020) Surface functionalized Casuarina equisetifolia pine powder for the removal of hetero-polyaromatic dye: characteristics and adsorption. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1798418
Cabrera-Codony A, Gonzalez-Olmos R, Martín MJ (2015) Regeneration of siloxane-exhausted activated carbon by advanced oxidation processes. J Hazard Mater 285:501–508. https://doi.org/10.1016/j.jhazmat.2014.11.053
Santos DHS, Duarte JLS, Tonholo J et al (2020) Saturated activated carbon regeneration by UV-light, H2O2 and Fenton reaction. Sep Purif Technol 250:117112. https://doi.org/10.1016/j.seppur.2020.117112
Ma Y (2017) Comparison of activated carbons prepared from wheat straw via ZnCl2 and KOH activation. Waste Biomass Valorization 8:549–559. https://doi.org/10.1007/s12649-016-9640-z
Nasrullah A, Saad B, Bhat AH et al (2019) Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: characterization and application for methylene blue removal. J Clean Prod 211:1190–1200. https://doi.org/10.1016/j.jclepro.2018.11.094
Oginni O, Singh K, Oporto G et al (2019) Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresour Technol Rep 7:100266. https://doi.org/10.1016/j.biteb.2019.100266
Zhang X, Zhang L, Li A (2018) Eucalyptus sawdust derived biochar generated by combining the hydrothermal carbonization and low concentration KOH modification for hexavalent chromium removal. J Environ Manage 206:989–998. https://doi.org/10.1016/j.jenvman.2017.11.079
Baloo L, Isa MH, Bin SN et al (2021) Adsorptive removal of methylene blue and acid orange 10 dyes from aqueous solutions using oil palm wastes-derived activated carbons. Alexandria Eng J 60:5611–5629. https://doi.org/10.1016/j.aej.2021.04.044
El-Bery HM, Saleh M, El-Gendy RA et al (2022) High adsorption capacity of phenol and methylene blue using activated carbon derived from lignocellulosic agriculture wastes. Sci Rep 12:1–17. https://doi.org/10.1038/s41598-022-09475-4
Pimentel CH, Freire MS, Gómez-Díaz D, González-Álvarez J (2023) Removal of wood dyes from aqueous solutions by sorption on un-treated pine sawdust. Proc 17th Int Conf Environ Sci Technol 17:. https://doi.org/10.30955/gnc2021.00438
Jawad AH, Malek NNA, Khadiran T et al (2022) Mesoporous high-surface-area activated carbon from biomass waste via microwave-assisted-H3PO4 activation for methylene blue dye adsorption: an optimized process. Diam Relat Mater 128:109288. https://doi.org/10.1016/j.diamond.2022.109288
Pimentel CH, Díaz-Fernández L, Gómez-Díaz D et al (2023) Separation of CO2 using biochar and KOH and ZnCl2 activated carbons derived from pine sawdust. J Environ Chem Eng 11:1–16. https://doi.org/10.1016/j.jece.2023.111378
Malik PK (2004) Dye removal from wastewater using activated carbon developed from sawdust: adsorption equilibrium and kinetics. J Hazard Mater 113:81–88. https://doi.org/10.1016/j.jhazmat.2004.05.022
Grimm A, dos Reis GS, Khokarale SG et al (2023) Shiitake spent mushroom substrate as a sustainable feedstock for developing highly efficient nitrogen-doped biochars for treatment of dye-contaminated water. J Water Process Eng 56:. https://doi.org/10.1016/j.jwpe.2023.104435
Boulanger N, Talyzin AV, Xiong S et al (2024) High surface area activated carbon prepared from wood-based spent mushroom substrate for supercapacitors and water treatment. Colloids Surf A Physicochem Eng Asp 680:132684. https://doi.org/10.1016/j.colsurfa.2023.132684
Álvarez-Torrellas S, Muñoz M, Zazo JA et al (2016) Synthesis of high surface area carbon adsorbents prepared from pine sawdust-Onopordum acanthium L. for nonsteroidal anti-inflammatory drugs adsorption. J Environ Manage 183:294–305. https://doi.org/10.1016/j.jenvman.2016.08.077
Liu X, Tian J, Li Y et al (2019) Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J Hazard Mater 373:397–407. https://doi.org/10.1016/j.jhazmat.2019.03.103
Spessato L, Bedin KC, Cazetta AL et al (2019) KOH-super activated carbon from biomass waste: insights into the paracetamol adsorption mechanism and thermal regeneration cycles. J Hazard Mater 371:499–505. https://doi.org/10.1016/j.jhazmat.2019.02.102
Bazan-Wozniak A, Cielecka-Piontek J, Nosal-Wiercińska A, Pietrzak R (2022) Adsorption of organic compounds on adsorbents obtained with the use of microwave heating. Materials (Basel) 15:. https://doi.org/10.3390/ma15165664
Khadir A, Muthu SS (2022) Polymer technology in dye-containing wastewater. Springer
Giles CH, MacEwan T, Nakhwa S, Smith D (1960) A system of classification of solution adsorption isotherms and its use in diagnosis of adsorption mechanisms and in measurement of specific surface area solids. J Chem Soc 14:3973–3993
Baysal M, Bilge K, Yılmaz B et al (2018) Preparation of high surface area activated carbon from waste-biomass of sunflower piths: kinetics and equilibrium studies on the dye removal. J Environ Chem Eng 6:1702–1713. https://doi.org/10.1016/j.jece.2018.02.020
Aljeboree AM, Alshirifi AN, Alkaim AF (2017) Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab J Chem 10:S3381–S3393. https://doi.org/10.1016/j.arabjc.2014.01.020
Aygün A, Yenisoy-Karakaş S, Duman I (2003) Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater 66:189–195. https://doi.org/10.1016/j.micromeso.2003.08.028
González-García P (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sustain Energy Rev 82:1393–1414. https://doi.org/10.1016/j.rser.2017.04.117
Manera C, Tonello AP, Perondi D, Godinho M (2019) Adsorption of leather dyes on activated carbon from leather shaving wastes: kinetics, equilibrium and thermodynamics studies. Environ Technol (United Kingdom) 40:2756–2768. https://doi.org/10.1080/09593330.2018.1452984
Amin MT, Alazba AA (2017) Comparative study of the absorptive potential of raw and activated carbon Acacia nilotica for Reactive Black 5 dye. Environ Earth Sci 76:1–13. https://doi.org/10.1007/s12665-017-6927-8
Belayachi H, Bestani B, Benderdouche N, Belhakem M (2019) The use of TiO2 immobilized into grape marc-based activated carbon for RB-5 Azo dye photocatalytic degradation. Arab J Chem 12:3018–3027. https://doi.org/10.1016/j.arabjc.2015.06.040
Tran HN, Wang YF, You SJ, Chao HP (2017) Insights into the mechanism of cationic dye adsorption on activated charcoal: the importance of Π-Π interactions. Process Saf Environ Prot 107:168–180. https://doi.org/10.1016/j.psep.2017.02.010
da Santos DH, S, Xiao Y, Chaukura N, et al (2022) Regeneration of dye-saturated activated carbon through advanced oxidative processes: a review. Heliyon 8:e10205. https://doi.org/10.1016/j.heliyon.2022.e10205
Acknowledgements
Authors would like to thank the use of RIAIDT-USC analytical facilities.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the Ministerio de Ciencia, Innovación y Universidades, grant number PGC2018-101047-B-I00.
Author information
Authors and Affiliations
Contributions
Catarina H. Pimentel: investigation, data curation, validation, visualization, writing—original draft. Rubén Castro Agra: investigation, data curation, validation, conducted the experiments, and data/evidence collection. Diego Gómez-Díaz: conceptualization, methodology, supervision, validation, funding acquisition, writing—review and editing. M. Sonia Freire: conceptualization, methodology, supervision, validation, funding acquisition, writing—review and editing. Julia González-Álvarez: conceptualization, methodology, supervision, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
All authors agreed to submit this research work for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pimentel, C.H., Castro-Agra, R., Freire, M.S. et al. Adsorption of anionic wood dyes on KOH-activated carbons from Pinus radiata sawdust. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05587-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05587-1