Abstract
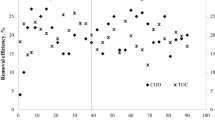
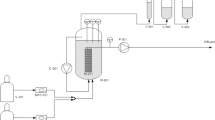
While developing a low-sulphate system combining indirect chromate-reduction by biologically-produced hydrogen sulphide and direct biological chromate-reduction to treat chromate-bearing waters, the aim of the present work was to evaluate the influence of sulphate and H2 starvation on chromate reduction. Chromate-reduction was performed under continuous-feed conditions in a fixed-film column bioreactor originally inoculated with a bacterial consortium containing Desulfomicrobium norvegicum, and fed with H2. With 500 mg l−1 of sulphate in the feed solution, total chromate-reduction was observed in the effluent whereas sulphate-reduction was strongly decreased, as also confirmed by measurements of isotopic ratios for sulphur. In the absence of sulphate, a chromate-reduction activity was still observed but was lower than in the presence of sulphate, and chromate-reduction was H2-dependent. Molecular biology techniques revealed the composition of the bacterial population in the effluent. D. norvegicum together with other micro-organisms of the Bacteria domain were detected. They include members related to the genera Acinetobacter, Acetobacterium and Rhodocyclus. Even when sulphate-reduction was strongly decreased, the presence of sulphate enhances the efficiency of the H2-dependent chromate-reduction. A H2- and CO2-consuming bacterial population may be used in a globally autotrophic process to reduce chromate at low sulphate concentration, thus avoiding excess sulphide production.





Similar content being viewed by others
References
Arias, Y. M., & Tebo, B. M. (2003). Cr(VI) reduction by sulphidogenic and non-sulphidogenic microbial consortia. Applied and Environmental Microbiology, 69, 1847–1853.
Battaglia-Brunet, F., Touze, S., Michel, C., & Ignatiadis, I. (2006). Treatment of a chromate-polluted groundwater in a 200-dm3 pilot bio-reactor fed with hydrogen. Journal of Chemical Technology and Biotechnology, 81, 1506–1513.
Battaglia-Brunet, F., Foucher, S., Denamur, A., Margraff, M., Morin, D., & Ignatiadis, I. (2004a). Chromate reduction at low sulphate concentration in hydrogen-fed bioreactors. Environmental Technology, 25(1), 101–109.
Battaglia-Brunet, F., Foucher, S., Morin, D., & Ignatiadis, I. (2004b). Chromate (\( {\rm CrO}^{{2 - }}_{4} \)) reduction in ground waters by using reductive bacteria in fixed-bed bioreactors. Water, Air & Soil Pollution, Focus, 4(4–5), 127–135.
Battaglia-Brunet, F., Foucher, S., Ignatiadis, I., Michel, C., & Morin, D. (2002). Reduction of chromate by fixed films of sulphate-reducing bacteria using hydrogen as electron source. Journal of Industrial Microbiology & Biotechnology, 28, 154–159.
Benson, D., Boguski, M. S., Lipman, D. J., Ostell, J., Ouellette, B. F., Rapp, B. A., et al. (1999). GenBank. Nucleic Acids Research, 27, 12–17.
Bhide, J. V., Dhakephalkar, P. K., & Paknikar, K. M. (1996). Microbiological process for the removal of Cr(VI) from chromate-bearing cooling tower effluent. Biotechnology Letters, 18, 667–672.
Blasiak, J., & Kowalik, J. (2000). A comparison of the in vitro genotoxicity of tri- and hexavalent chromium. Mutation Research, 469, 135–145.
Böttcher, M. E., Brumsack, H. J., & De Lange, G. J. (1998a). Sulphate reduction and related stable isotope (34S, 18O) variations in interstitial waters from the Eastern Mediterranean. Proceedings of Ocean Drilling Program, Scientific Results, 160, 365–373.
Böttcher, M. E., Oelshläger, B., Höpner, T., Brumsack, H. J., & Rullkötter, J. (1998b). Sulphate reduction related to the early diagenesic degradation of organic matter and “black spot” formation in tidal sandflats of the German Wadden Sea (Southern North Sea): Stable isotope (13C, 34S, 18O) and other geochemical results. Organic Geochemistry, 29, 1517–1560.
Caron, F., Tessier, A., Kramer, J. R., Schwarcz, H. P., & Rees, C. E. (1986). Sulphur and oxygen isotopes of sulphate in precipitation and lakewater, Quebec, Canada. Applied Geochemistry, 1, 601–606.
Cervantes, C. (1991). Bacterial interaction with chromate. Antonie van Leeuwenhoek, 59, 229–233.
Chardin, B., Giudici-Orticoni, M. T., De Luca, G., Guigliarelli, B., & Bruschi, M. (2003). Hydrogenases in sulphate-reducing bacteria function as chromium reductase. Applied Microbiology and Biotechnology, 63, 315–321.
Cheung, K. H., & Gu, J. D. (2003). Reduction of chromate (\( {\rm CrO}^{{2 - }}_{4} \)) by an enrichment consortium and an isolate of marine sulphate-reducing bacteria. Chemosphere, 52, 1523–1529.
Chulsung, K., Gunhui, Z., Baolin, D., Thornton, E. C., & Huifang, X. (2001). Chromium(VI) reduction by hydrogen sulphide in aqueous media: Stoichiometry and kinetics. Environmental Science and Technology, 35, 2219–2225.
Cole, J. R., Chai, B., Marsh, T. L., Farris, R. J., Wang, Q., Kulam, S. A., et al. (2003). The Ribosomal Database Project (RDP-II): Previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Research, 31, 442–443.
Dilek, F. B., & Gökçay, C. F. (1996). Microbiology of activated sludge treating wastewater containing Ni(II) and Cr(VI). Water Science and Technology, 34, 183–191.
Epstein, S. & Mayeda, T. (1953). Variation of 18O content of waters from natural sources. Geochimica et Cosmochimica Acta, 4, 213–224.
Fauque, G., & Ollivier, O. (2003). Anaerobes: The sulphate-reducing bacteria as an example of metabolic diversity. In A. T. Bull (Ed.) Microbial Diversity and Bioprospecting (vol. 17, pp. 169–176). Washington: ASM.
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39, 783–791.
Francisco, R., Alpoim, M. C., & Morais, P. V. (2002). Diversity of chromium-resistant and -reducing bacteria in a chromium-contaminated activated sludge. Journal of Applied Microbiology, 92, 837–843.
Gruber, J. E., & Jennette, K. W. (1978). Metabolism of the carcinogen chromate by rat liver microsomes. Biochemical and Biophysical Research Communications, 82, 700–706.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Horita, J., Ueda, A., Mizukami, K., & Takatori, I. (1989). Automatic δD and δ18O analyses of multi-water samples using H2- and CO2-water equilibration methods with a common equilibration set-up. Applied Radiation and Isotopes, 40, 801–805.
Joulian, C., Ramsing, N. B., & Ingvorsen, K. (2001). Congruent phylogenies of most common small-subunit rRNA and dissimilatory sulphite reductase gene sequences retrieved from estuarine sediments. Applied and Environmental Microbiology, 67, 3314–3318.
Jukes, T. H., & Cantor, C. R. (1969). Evolution of protein molecules. In H. N. Munro (Ed.) Mammalian Protein Metabolism (pp. 211–232). New York: Academic.
Kim, C., Zhou, Q., Deng, B., Thornton, E., & Xu, H. (2001). Chromium reduction by hydrogen sulphide in aqueous media: Stoichiometry and kinetics. Environmental Science and Technology, 35, 2219–2225.
Kimura, M. (1980). A simple model for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120.
Krumholz, L. R., Harris, S. H., Tay, S. T., & Sulfita, J. M. (1999). Characterization of two subsurface H2-utilizing bacteria, Desulfomicrobium hypogeium sp. nov. and Acetobacterium psammolithicum sp. nov. and their ecological roles. Applied and Environmental Microbiology, 65, 2300–2306.
Lojou, E., Bianco, P., & Bruschi, M. (1998). Kinetic studies on the electron transfer between bacterial c-type cytochromes and metal oxides. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 452, 167–177.
Lomans, B. P., Leijdekkers, P., Wesselink, J.-J., Bakkes, P., Pol, A., van der Drift, C., et al. (1991). Obligate sulphide-dependent degradation of methoxylated aromatic compounds and formation of methanethiol and dimethyl sulphide by a freshwater sediment isolate, Parasporobacterium paucivorans gen. nov., sp. nov. Applied and Environmental Microbiology, 67, 4017–4023.
Lovley, D. R. (1994). Microbial reduction of iron, manganese and other metals. Advances in Agronomy, 54, 175–229.
Lovley, D. R., & Phillips, E. J. P. (1994). Reduction of chromate by Desulfovibrio vulgaris and its c3 cytochrome. Applied and Environmental Microbiology, 60, 726–728.
Lupton, F. S., DeFilippi, L. J., & Goodman, J.R. (1991). Bioremediation of chromium (VI) contaminated aqueous systems by sulphate reducing bacteria. US patent n° 5,062,956.
Michel, C., Brugna, M., Aubert, C., Bernadac, A., & Bruschi, M. (2001). Enzymatic reduction of chromate: Comparative studies using sulphate-reducing bacteria. Key role of polyheme cytochromes c and hydrogenases. Applied Microbiology and Biotechnology, 55, 95–100.
Pettine, M., Millero, F. J., & Passiro, R. (1994). Reduction of chromium (VI) with hydrogen sulphide in NaCl media. Marine Chemistry, 46, 335–344.
Rai, D., Moore, D. A., Hess, N. J., Rao, L., & Clark, S. B. (2004). Chromium(III) hydroxide solubility in the aqueous \({\text{Na}}^{ + } - {\text{OH}}^{ - } - {\text{H}}_{2} {\text{PO}}^{ - }_{4} - {\text{HPO}}^{{2 - }}_{4} - {\text{PO}}^{3}_{4} - {\text{H}}_{2} {\text{O}}\) system. A thermodynamic model. Journal of Solution Chemistry, 33, 1213–1242.
Rai, D., Sass, B. M., & Moore, D. A. (1987). Chromium (III) hydrolysis constants and solubility of chromium (III) hydroxide. Inorganic Chemistry, 26, 345–349.
Saitou, N., & Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 405–425.
Sakai, H., & Krouse, H. R. (1971). Elimination of memory effects in 18O–16O determination in sulphates. Earth and Planetary Science Letters, 11, 361–373.
Smith, W., & Gadd, G. (2000). Reduction and precipitation of chromate by mixed culture sulphate-reducing bacterial biofilms. Journal of Applied Microbiology, 88, 983–991.
Sinha, S. N., & Barnerjee, R. D. (1997). Ecological role of thiosulphate and sulphide utilizing purple nonsulphur bacteria of a riverine ecosystem. FEMS Microbiology Ecology, 24, 211–220.
Tebo, B. M., & Obraztsova, A. Y. (1998). Sulphate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiology Letters, 162, 193–198.
Thode, H. G., Monster, J., & Dunford, H. B. (1961). Sulphur isotope geochemistry. Geochimica and Cosmochimica Acta, 25, 50–174.
Thomsen, T. R., Finster, K., & Ramsing, N. B. (2001). Biogeochemical and molecular signatures of anaerobic methane oxidation in a marine sediment. Applied and Environmental Microbiology, 67, 1646–1656.
Turick, C. E., & Appel, W. A. (1997). Method for in situ or ex situ bioremediation of hexavalent chromium contaminated soils and/or groundwater. US Patent n° 5,681,739.
Turpeinen, R., Kairesalo, T., & Häggblom, M. M. (2004). Microbial community structure and activity in arsenic-, chromium- and copper- contaminated soils. FEMS Microbiology Ecology, 47, 39–50.
Vainshtein, M., Kuschk, P., Mattusch, J., Vatsourina, A., & Wiessner, A. (2003). Model experiments on the microbial removal of chromium from contaminated groundwater. Water Research, 37, 1401–1405.
Van de Peer, Y., & De Wachter, R. (1994). TREECON for Windows: A software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Computer Applications in the Biosciences, 10, 569–570.
Wagner, M., Roger, A., Flax, J., Brusseau, G. & Stahl, D. (1998). Phylogeny of dissimilatory sulphite reductases supports an early origin of sulphate respiration. Journal of Bacteriology, 180, 2975–2982.
Wang, Y. T. (2000). Microbial reduction of chromate. In D. R. Lovley (Ed.), Environmental microbe-metal interactions, chap. 10 (pp. 225–235). Washington D.C.: ASM.
Zilles, J. L., Peccia, J., Kim, M. W., Hung, C. H., & Noguera, D. R. (2002). Involvement of Rhodocyclus-related organisms in phosphorus removal in full-scale wastewater treatment plants. Applied and Environmental Microbiology, 68, 2763–2769.
Acknowledgements
This is the BRGM contribution n° 02811. This work was carried out in the framework of the Commission of European Union contract no EVK1-CT-1999-00033 (METALBIOREDUCTION project) and received financial support from a BRGM research project (BIOPROC project). We thank M. Marggraff from Munters-Euroform for providing filling materials and C. Flehoc from Metrology, Monitoring and Analysis Division, BRGM/MMA, for the isotopic determinations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Battaglia-Brunet, F., Michel, C., Joulian, C. et al. Relationship Between Sulphate Starvation and Chromate Reduction in a H2-fed Fixed-film Bioreactor. Water Air Soil Pollut 183, 341–353 (2007). https://doi.org/10.1007/s11270-007-9383-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9383-3