Abstract
Tobacco rattle virus (TRV) is an important soil-borne virus of potato that is transmitted by stubby-root nematodes. TRV causes corky ringspot, a tuber disease of economic importance to potato production. Utilizing protein-coding regions of the whole genome and a range of computational tools, the genetic diversity, and population structure of TRV isolates from several potato-growing regions (Colorado, Idaho, Indiana, Minnesota, Nebraska, North Dakota, and Washington State) in the USA were determined. Phylogenetic analyses based on RNA2 nucleotide sequences, the coat protein (CP) and nematode transmission (2b) genes, showed geographical clustering of USA isolates with previously known American isolates, while European isolates grouped in a distinct cluster. This was corroborated by the observed genetic differentiation and infrequent gene flow between American and European isolates. Low genetic diversity was revealed among American isolates compared to European isolates. Phylogenetic clustering based on RNA1 genes (RdRp, RdRp-RT, and 1a) were all largely incongruent to that of 1b gene (virus suppressor of RNA silencing). This genetic incongruence suggested the influence of recombination. Furthermore, the RdRp, RdRp-RT, and 1a genes were predicted to be more conserved and under negative selection, while the 1b gene was less constrained. Different evolutionary lineages between TRV RNA1 and RNA2 genomic segments were revealed.



Similar content being viewed by others
References
Adams MJ, Adkins S, Bragard C, Gilmer D, Li D, MacFarlane SA, Wong SM, Melcher U, Ratti C, Ryu KH (2017) ICTV virus taxonomy profile: Virgaviridae. J Gen Virol 98:1999–2000. https://doi.org/10.1099/jgv.0.000884
Adams MJ, Antoniw JF, Kreuze J (2009) Virgaviridae: a new family of rod-shaped plant viruses. Arch Virol 154:1967–1972. https://doi.org/10.1007/s00705-009-0506-6
MacFarlane SA (1999) Molecular biology of the tobraviruses. J Gen Virol 80:2799–2807. https://doi.org/10.1099/0022-1317-80-11-2799
Ashfaq M, McGavin W, MacFarlane SA (2011) RNA2 of TRV SYM breaks the rules for tobravirus genome structure. Virus Res 160:435–438. https://doi.org/10.1016/j.virusres.2011.07.007
Mojtahedi H, Santo GS (1999) Ecology of Paratrichodorus allius and its relationship to the corky ring-spot disease of potato in the Pacific Northwest. Am J Potato Res 76:273–280. https://doi.org/10.1007/bf02853625
Pérez EE, Weingartner DP, Hiebert E, McSorley R (2000) Tobacco rattle virus detection in potato tubers from northeast Florida by PCR and tissue blotting. Am J Potato Res 77:363–368. https://doi.org/10.1007/bf02882290
Xenophontos S, Robinson DJ, Dale MFB, Brown DJF (1998) Evidence for persistent, symptomless infection of some potato cultivars with tobacco rattle virus. Potato Res 41:255–265. https://doi.org/10.1007/BF02358195
Crosslin JM, Hamm PB, Kirk WW, Hammond RW (2010) Complete genomic sequence of a tobacco rattle virus isolate from Michigan-grown potatoes. Arch Virol 155:621–625. https://doi.org/10.1007/s00705-010-0609-0
Weingartner DP, Shumaker JR (1990) Control of nematodes and soil-borne diseases in florida potatoes with aldicarb and 1,3-d. J Nematol 22:775–778
Mojtahedi H, Crosslin JM, Santo GS, Brown CR, Thomas PE (2001) Pathogenicity of Washington and Oregon isolates of tobacco rattle virus on potato. Am J Potato Res 78:183–190. https://doi.org/10.1007/BF02883543
Crosslin JM, Thomas PE, Hammond RW (2003) Genetic variability of genomic RNA 2 of four tobacco rattle tobravirus isolates from potato fields in the Northwestern United States. Virus Res 96:99–105. https://doi.org/10.1016/S0168-1702(03)00177-1
Gudmestad NC, Mallik I, Pasche JS, Crosslin JM (2008) First report of tobacco rattle virus causing corky ringspot in potatoes grown in Minnesota and Wisconsin. Plant Dis 92:1254–1254. https://doi.org/10.1094/pdis-92-8-1254c
Mojtahedi H, Boydston RA, Thomas PE, Crosslin JM, Santo GS, Riga E, Anderson TL (2003) Weed Hosts of Paratrichodorus allius and tobacco rattle virus in the Pacific Northwest. Am J Potato Res 80:379–385. https://doi.org/10.1007/BF02854249
David N, Mallik I, Gudmestad NC (2010) First report of tobacco rattle virus associated with corky ringspot in potatoes grown in North Dakota. Plant Dis 94:130–130. https://doi.org/10.1094/PDIS-94-1-0130B
MacFarlane SA, Vassilakos N, Brown DJF (1999) Similarities in the genome organization of tobacco rattle virus and pea early-browning virus isolates that are transmitted by the same vector nematode. J Gen Virol 80:273–276. https://doi.org/10.1099/0022-1317-80-1-273
Yin Z, Pawełkowicz M, Michalak K, Chrzanowska M, Zimnoch-Guzowska E (2014) Single-nucleotide polymorphisms and reading frame shifts in RNA2 recombinant regions of tobacco rattle virus isolates Slu24 and Deb57. Arch Virol 159:3119–3123. https://doi.org/10.1007/s00705-014-2128-x
Drake JW, Holland JJ (1999) Mutation rates among RNA viruses. Proc Natl Acad Sci USA 96:13910–13913. https://doi.org/10.1073/pnas.96.24.13910
Domingo E (2000) Viruses at the edge of adaptation. Virology 270:251–253. https://doi.org/10.1006/viro.2000.0320
Koenig R, Lesemann DE, Pleij CWA (2012) Tobacco rattle virus genome alterations in the Hosta hybrid “Green Fountain” and other plants: reassortments, recombinations and deletions. Arch Virol 157:2005–2008. https://doi.org/10.1007/s00705-012-1365-0
Koenig R, Lesemann DE, Pfeilstetter E, Winter S, Pleij CWA (2011) Deletions and recombinations with the RNA1 3′ ends of different tobraviruses have created a multitude of tobacco rattle virus TCM-related RNA2 species in Alstroemeria and tulip. J Gen Virol 92:988–996. https://doi.org/10.1099/vir.0.028803-0
Robinson DJ (2004) Identification and nucleotide sequence of a tobacco rattle virus RNA-1 variant that causes spraing disease in potato cv. Bintje. J Phytopathol 152:286–290. https://doi.org/10.1111/j.1439-0434.2004.00842.x
Elena SF, Fraile A, García-Arenal F (2014) Evolution and emergence of plant viruses. Adv Virus Res 88:161–191. https://doi.org/10.1016/b978-0-12-800098-4.00003-9
Kozyreva NI, Romanenko ND (2008) Distribution of the nematodes from family Trichodoridae, vectors of the tobacco rattle virus, in the Moscow Oblast. Parazitologiia 42:428–434
Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM (2014) The evolution and genetics of virus host shifts. PLoS Pathog 10:e1004395. https://doi.org/10.1371/journal.ppat.1004395
Robinson DJ, Dale MFB, Todd D (2004) Factors affecting the development of disease symptoms in potatoes infected by tobacco rattle virus. Eur J Plant Pathol 110:921–928. https://doi.org/10.1007/s10658-004-8950-3
Lafforgue G, Martinez F, Sardanyes J, de la Iglesia F, Niu Q-W, Lin S-S, Sole RV, Chua N-H, Daros J-A, Elena SF (2011) Tempo and mode of plant RNA virus escape from RNA interference-mediated resistance. J Virol 85:9686–9695. https://doi.org/10.1128/jvi.05326-11
Martín-Hernández AM, Baulcombe DC (2008) Tobacco rattle virus 16-kilodalton protein encodes a suppressor of RNA silencing that allows transient viral entry in meristems. J Virol 82:4064–4071. https://doi.org/10.1128/jvi.02438-07
Martínez-Priego L, Donaire L, Barajas D, Llave C (2008) Silencing suppressor activity of the tobacco rattle virus-encoded 16-kDa protein and interference with endogenous small RNA-guided regulatory pathways. Virology 376:346–356. https://doi.org/10.1016/j.virol.2008.03.024
Andika IB, Kondo H, Nishiguchi M, Tamada T (2012) The cysteine-rich proteins of beet necrotic yellow vein virus and tobacco rattle virus contribute to efficient suppression of silencing in roots. J Gen Virol 93:1841–1850. https://doi.org/10.1099/vir.0.043513-0
Elena SF (2017) Local adaptation of plant viruses: lessons from experimental evolution. Mol Ecol 26:1711–1719. https://doi.org/10.1111/mec.13836
Acknowledgements
We thank Dr. Prabu Gnanasekaran for a critical review of the manuscript. The research was supported by USDA-NIFA-SCRI Grant Number 2014-51181-22373, USDA National Institute of Food and Agriculture, Hatch project Accession #1016563 “Reducing the Impact of Pests and Diseases Affecting Washington Agriculture.” LM acknowledges support from a Fulbright fellowship and a 2016 Fulbright research grant award, Graduate Research Assistantships from the Graduate Program in Molecular Plant Sciences, and the Department of Plant Pathology at Washington State University (WSU), Pullman, WA, 99164, USA. LM also acknowledges a study-leave from the National University of Science and Technology, Bulawayo, Zimbabwe.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving human and/or animals participants
Not applicable.
Informed consent
Not applicable.
Additional information
Edited by Karel Petrzik.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 72 kb)
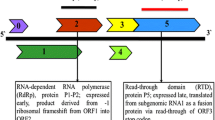
Supplementary Figure S1 (a) The schematic representation of the genome organization of tobacco rattle virus (TRV) RNA1 (6791 nucleotides). Predicted open reading frames are represented by boxes. The sequences of different TRV isolates have sequence similarity both at 5′ and 3′ ends. Hence the primers used were based on previous studies of Crosslin (2010). The sequences of primers for complete RNA1 amplification are TRV_R1-F 5′-ATAAAACATTTCAATCCTTTGAACG-3′ and TRVR1-R 5′-GGGCGTAATAACGCTTACGTAG-3′. Along with these primers for complete genome amplification, a pair of primer was designed based on previous studies for amplification of 463bp fragment near the 3′ end of 16KDa gene of TRV RNA1. The primer pair is as follows: TRV_463-F 5′-CAGTCTATACACAGAAACAGA-3′ and TRVR1-R 5′-GACGTGTGTACTCAAGGGTT-3′. (b) Primers were designed to allow for amplification of TRV RNA1 as overlapping fragments, A-B (2.8 kb), C-D (1.6 kb) and E-F (2.3 kb). For the 2.8 kb fragment, primer pairs used are TRVR1-2F 5′-ATAAAACATTTCAATCCTTTGAACG-3′ and TRVRNA-2R 5′-TTGTCCAAGATCAACCTGTTAT-3′, for the 1.6 kb fragment primer pairs used are TRV1-3F 5′-ATAACAGGTTGATCTTGGACAA-3′ and TRV-3R TGGTTGCATAGCATCCAACTTG-3′ while for the 2.3 kb fragment primer pairs used are TRV1RNA-4F 5′-CAAGTTGGATGCTATGCAACCA-3′ and RNA-1R GGGCGTAACGCTTACGTAG-3′. Internal sequencing primers were designed to get the complete genome of TRV RNA1.
Supplementary file2 (TIF 302 kb)
Supplementary Figure S2 (a) Almost the complete sequence of RNA2 was amplified by using primer TR1 and 158 (I and J). Primer TR1 is 5′-GTTGGAGAACGCGGTAGA-3` and 158 is 5′-GGGCGTAATAACGCTTACG-3′. TR1 corresponds to the sequence at position 75–92 in RNA2 which is highly conserved in tobacco rattle virus (TRV) isolates (Schmitt et al., 1998); 158 is complementary to the 19 3′-terminal nucleotides of RNA2 of TRV isolates (MacFarlane, 1996). The forward primer at position G is R2-4 5′-ATAAAACATTGCACCWWTGGTGTTGC-3′ and reverse primer at position H is R2-3 5′-CGTAATAACGCTTACGTAGGCGAG-3′. MI-2 isolate (GenBank accession: GQ903772) is representative sequence showing complete RNA2 genome of TRV along with the locations of primers used in present studies. The 5′ region was found to be highly conserved among various TRV isolates when compared to the 3′ region. The internal sequencing primers were designed to sequence the complete amplicon of TRV RNA2. (b) The schematic diagram of RNA2 from different TRV isolates that were amplified and sequenced in the present studies.
Supplementary file3 (TIF 244 kb)
Supplementary Figure S3 Schematic representation of genome organization of tobacco rattle virus (TRV) RNA2 of six different isolates that are previously reported. Yellow colored boxes represent CP; Red color – 37 KDa/2b protein and blue colored boxes – 2c/18KDa/34KDa protein. Boxes with dash denote truncated ORFs. Downward arrow indicates start of sequence identical to the 3′-end of the RNA1. The sequences of different TRV isolates have sequence similarity both at 5′ and 3′ ends. Hence the primers used were based on previous studies of Crosslin (2010). The sequences of primers for complete RNA2 amplification is R2-4 5′-ATAAAACATTGCACCWWTGGTGTTGC-3′ and R2-3 5′-CGTAATAACGCTTACGTAGGCGAG-3′.
Supplementary file4 (DOCX 17 kb)
Supplementary Table S1 Evidence of breakpoints and recombination in tobacco rattle virus (TRV) RNA1 isolates.
Supplementary file5 (DOCX 17 kb)
Supplementary Table S2 Genetic differentiation and gene flow amongst tobacco rattle virus (TRV) populations for RNA1 and RNA2 gene sequences.
Supplementary file6 (DOCX 14 kb)
Supplementary Table S3 Neutrality tests in populations of tobacco rattle virus (TRV) based on RNA1 and RNA2 gene sequences.
Rights and permissions
About this article
Cite this article
Moyo, L., Raikhy, G., Hamid, A. et al. Phylogenetics of tobacco rattle virus isolates from potato (Solanum tuberosum L.) in the USA: a multi-gene approach to evolutionary lineage. Virus Genes 58, 42–52 (2022). https://doi.org/10.1007/s11262-021-01875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-021-01875-4