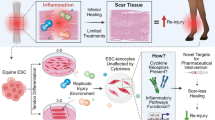
Abstract
Tendons regenerate poorly due to a dense extracellular matrix and low cellularity. Cellular therapies aim to improve tendon repair using mesenchymal stem cells and tenocytes; however, a current limitation is the low proliferative potential of tenocytes in cases of severe trauma. The purpose of this study was to develop a method useful in veterinary medicine to improve the differentiation of Peripheral Blood equine mesenchymal stem cells (PB-MSCs) into tenocytes. PB-MSCs were used to study the effects of the addition of some growth factors (GFs) as TGFβ3 (transforming growth factor), EGF2 (Epidermal growth factor), bFGF2 (Fibroblast growth factor) and IGF-1 (insulin-like growth factor) in presence or without Low Level Laser Technology (LLLT) on the mRNA expression levels of genes important in the tenogenic induction as Early Growth Response Protein-1 (EGR1), Tenascin (TNC) and Decorin (DCN). The singular addition of GFs did not show any influence on the mRNA expression of tenogenic genes whereas the specific combinations that arrested cell proliferation in favour of differentiation were the following: bFGF2 + TGFβ3 and bFGF2 + TGFβ3 + LLLT. Indeed, the supplement of bFGF2 and TGFβ3 significantly upregulated the expression of Early Growth Response Protein-1 and Decorin, while the use of LLLT induced a significant increase of Tenascin C levels. In conclusion, the present study might furnish significant suggestions for developing an efficient approach for tenocyte induction since the external administration of bFGF2 and TGFβ3, along with LLLT, influences the differentiation of PB-MSCs towards the tenogenic fate.



Similar content being viewed by others
References
Abate M, Di Gregorio P, Schiavone C, Salini V, Tosi U, Muttini A (2012) Platelet rich plasma in tendinopathies: how to explain the failure. Int J Immunopath Ph 25:325–334
Anitua E, de la Fuente M, Muruzabal F, Riestra A, Merayo-Lloves J, Orive G (2015) Plasma rich in growth factors (PRGF) eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Exp Eye Res doi. doi:10.1016/j.exer.2015.02.016
Barberini DJ, Freitas NP, Magnoni MS, Maia L, Listoni AJ, Heckler MC, Sudano MJ, Golim MA, da Cruz L-AF, Amorim RM (2014) Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem Cell Res Ther 5:25
Barsby T, Bavin EP, Guest DJ (2014) Three-dimensional culture and transforming growth factor beta3 synergistically promote tenogenic differentiation of equine embryo-derived stem cells. Tissue Eng Part A 20:2604–2613
Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13:1219–1227
Brehm W, Burk J, Delling U, Gittel C, Ribitsch I (2012) Stem cell-based tissue engineering in veterinary orthopaedics. Cell Tissue Res 347:677–688
Broeckx S, Suls M, Beerts C, Vandenberghe A, Seys B, Wuertz-Kozak K, Duchateau L, Spaas JH (2014a) Allogenic mesenchymal stem cells as a treatment for equine degenerative joint disease: a pilot study. Curr Stem Cell Res Ther 9:497–503
Broeckx S, Zimmerman M, Crocetti S, Suls M, Mariën T, Ferguson SJ, Chiers K, Duchateau L, Franco-Obregón A, Wuertz K, Spaas JH (2014b) Regenerative therapies for equine degenerative joint disease: a preliminary study. PLoS One 9(1):e85917
Broeckx S, Borena BM, Zimmerman M, Mariën T, Seys B, Suls M, Duchateau L, Spaas JH (2014c) Intravenous application of allogenic peripheral blood-derived mesenchymal stem cells: a safety assessment in 291 equine recipients. Curr Stem Cell Res Ther 9:452–457
Brown GL, Curtsinger L, Jurkiewicz MJ, Nahai F, Schultz G (1991) Stimulation of healing of chronic wounds by epidermal growth factor. Plast Reconstr Surg 88:189–194
Cai TY, Zhu W, Chen XS, Zhou SY, Jia LS, Sun YQ (2013) Fibroblast growth factor 2 induces mesenchymal stem cells to differentiate into tenocytes through the MAPK pathway. Mol Med Rep 8:1323–1328
Chen MH, Huang YC, Sun JS, Chao YH, Chen MH (2015) Second messengers mediating the proliferation and collagen synthesis of tenocytes induced by low-level laser irradiation. Lasers Med Sci 30:263–272
Crovace A, Lacitignola L, Rossi G, Francioso E (2010) Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon. Vet Med Int. doi:10.4061/2010/250978
Delincé P, Ghafil D (2012) Anterior cruciate ligament tears: conservative or surgical treatment? Knee Surg Sports Traumatol Arthrosc 20:48–61
Dimauro I, Grasso L, Fittipaldi S, Fantini C, Mercatelli N, Racca S, Geuna S, Di Gianfrancesco A, Caporossi D, Pigozzi F, Borrione P (2014) Platelet-rich plasma and skeletal muscle healing: A molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS One 9(7):e:102993.
Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ (2013) Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol 32:3–13
Durgam SS, Stewart AA, Pondenis HC, Gutierrez-Nibeyro SM, Evans RB, Stewart MC (2012) Comparison of equine tendon- and bone marrow-derived cells cultured on tendon matrix with or without insulin-like growth factor-I supplementation. Am J Vet Res 73:153–161
Freedman BR, Gordon JA, Soslowsky LJ (2014) The Achilles tendon: fundamental properties and mechanisms governing healing. Muscles, Ligaments and Tendons J 4:245–255
Fu SC, Wang W, Pau HM, Wong YP, Chan KM, Rolf CG (2002) Increased expression of transforming growth factor-beta 1 in patellar tendinosis. Clin Orthop Relat R 400:174–183
Giovannini S, Brehm W, Mainil-Varlet P, Nesic D (2008) Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation 76:118–129
Goncalves AI, Rodrigues MT, Lee SJ, Atala A, Yoo JJ, Reis RL, Gomes M.E (2013) Understanding the role of growth factors in modulating stem cell tenogenesis. PLoS One 8:e83734.
Hamblin MR, Demidova TN (2006) Mechanisms of low level light therapy. doi:10.1117/12.646294.
Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT (2014) FOXO1, TGF-β regulation and wound healing. Int Mol Sci 15:16257–16269
Hansen M, Boesen A, Holm L, Flyvbjerg A, Langberg H, Kjaer M (2013) Local administration of insulin-like growth factor-I (IGF-I) stimulates tendon collagen synthesis in humans. Scand J Med Sci Sports 23:614–619
Hawkins D, Abrahamse H (2006) The role of laser fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon laser irradiation. Lasers Surg Med 38:74–83
Hessel LN, Bosch G, van Weeren PR, Ionita JC (2015) Equine autologous platelet concentrates: a comparative study between different available systems. Equine Vet J 47:319–325
Hoffmann A, Gross G (2007) Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches. Int Orthop 31:791–797
Hoynowski SM, Fry MM, Gardner BM, Leming MT, Tucker JR, Black L, Sand T, Mitchell KE (2007) Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem Bioph Res Co 19:347–353
Huertas RM, Luna-Bertos ED, Ramos-Torrecillas J, Leyva FM, Ruiz C, García-Martinez O (2014) Effect and clinical implications of the low-energy diode laser on bone cell proliferation. Biol Res Nur 16:191–196
Iacopetti I, Perazzi A, Maniero V, Martinello T, Patruno M, Glazar M, Busetto R (2015) Effect of MLS(®) laser therapy with different dose regimes for the treatment of experimentally induced tendinopathy in sheep: pilot study. Photomed Laser Surg 33:154–163
Jiang D, Xu B, Yang M, Zhao Z, Zhang Y, Li Z (2014) Efficacy of tendon stem cells in fibroblast-derived matrix for tendon tissue engineering. Cytotherapy 16:662–673
Khan U, Kakar S, Akali A, Bentley G, McGrouther DA (2000) Modulation of the formation of adhesions during the healing of injured tendons. J Bone Joint Surg Br Vol 82:1054–1058
Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J (2002) Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg 27:615–620
Koerner J, Nesic D, Romero JD, Brehm W, Mainil-Varlet P, Grogan SP (2006) Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells 24:1613–1619
Lejard V, Blais F, Guerquin MJ, Bonnet A, Bonnin MA, Havis E, Malbouyres M, Bidaud CB, Maro G, Gilardi-Hebenstreit P, Rossert J, Ruggiero F, Duprez D (2011) Egr1 and egr2 involvement in vertebrate tendon differentiation. J Biol Chem 286:5855–5867
Liu CF, Aschbacher-Smith I, Barthelery NJ, Dyment N, Butler D, Wylie C (2011) What we should know before using tissue engineering techniques to repair injured tendons: a developmental biology perspective. Tissue Eng. Part B Rev 17:165–176
Maia L, de Souza MV, Ribeiro Junior JI, de Oliveira AC, Silveira Alves GE, dos Anjos Benjamin L, Sancler Silva YFR, Mota Zandim B, do Carmo Lopes Moreira J (2009) Platelet-rich plasma in the treatment of induced tendinophaty in horses: histologic evaluation. J Equine Vet Sci 29:618–626.
Martinello T, Bronzini I, Maccatrozzo L, Iacopetti I, Sampaolesi M, Mascarello F, Patruno M (2010) Cryopreservation does not affect the stem characteristics of multipotent cells isolated from equine peripheral blood. Tissue Eng: Part C Methods 16:771–778
Martinello T, Bronzini I, Maccatrozzo L, Mollo A, Sampaolesi M, Mascarello F, Decaminada M, Patruno M (2011) Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res Vet Sci 91:18–24
Martinello T, Bronzini I, Perazzi A, Testoni S, De Benedictis GM, Negro A, Caporale G, Mascarello F, Iacopetti I, Patruno M (2013) Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J Orthop Res 2:306–314
Molloy T, Wang Y, Murrell G (2003) The roles of growth factors in tendon and ligament healing. Sports Med 33:381–394
Monici M, Cialdai F, Ranaldi F, Paoli P, Boscaro F, Moneti G, Caselli A (2013) Effect of IR laser on myoblasts: a proteomic study. Mol BioSyst 9:1147–1161.
Pajala A, Melkko J, Leppilahti J, Ohtonen P, Soini Y, Risteli J (2009) Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An Immunohistochemical sTudy Histol Histopathol 24:1207–1211
Patruno M, Martinello T (2014) Treatments of the injured tendon in veterinary medicine: from scaffolds to adult stem cells. Histol Histopathol 29:417–422
Pietschmann MF, Frankewycz B, Schmitz P, Docheva D, Sievers B, Jansson V, Schieker M, Müller PE (2013) Comparison of tenocytes and mesenchymal stem cells seeded on biodegradable scaffolds in a full-size tendon defect model. J Mater Sci Mater Med 24:211–220
Pires D, Xavier M, Araújo T, Silva Jr JA, Aimbire F, Albertini R (2011) Low-level therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 26:85–94
Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31:334–340
Pyo SJ, Song WW, Kim IR, Park BS, Kim CH, Shin SH, Chung IK, Kim YD (2013) Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-β1 in hypoxic-cultured human osteoblasts. Lasers Med Sci 28:543–550
Rider DA, Dombrowski C, Sawyer AA, Ng GH, Leong D, Hutmacher DW, Nurcombe V, Cool SM (2008) Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells 26:1598–1608
Riley G (2008) Tendinopathy from basic science to treatment. Nat Clin Pract Rheumatol 4:82–89
Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D (2015) Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat R 473:1635–1643
Schneider PR, Buhrmann C, Mobasheri A, Matis U, Shakibaei M (2011) Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J Orthop Res 29:1351–1360
Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J (2013) Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One 8:1–14
Spaas JH, Guest DJ, Van de Walle GR (2012) Tendon regeneration in human and equine athletes: Ubi sumus-Quo vadimus (where are we and where are we going to)? Sports Med 42:871–890
Spaas JH, De Schauwer C, Cornillie P, Meyer E, Van Soom A, Van de Walle GR (2013) Culture and characterisation of equine peripheral blood mesenchymal stromal cells. Vet J 195:107–113
Sperandio FF, Simões A, Corrêa L, Aranha AC, Giudice FS, Hamblin MR, Sousa SC (2014) Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J Biophotonics 20:9999
Takehara K (2000) Growth regulation of skin fibroblasts. J Dermatol Sci 24(Suppl 1):S70–S77
Tamama K, Fan V.H, Griffith LG, Blair HC, Wells A (2006) Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 24:686–695.
Tan Q, Lui PP, Rui YF (2012) Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells- implications in tissue engineering. Stem Cells Dev 20:790–800
Tang JB, Chen CH, Zhou YL, McKeever C, Liu PY (2014) Regulatory effects of introduction of an exogenous FGF2 gene on other growth factor genes in a healing tendon. Wound Repair Regen 22:111–118
Tao X, Liu J, Chen L, Zhou Y, Tang K (2015) EGR1 induces tenogenic differentiation of tendon stem cells and promotes rabbit rotator cuff repair. Cell Physiol Biochem 30:699–709
Toupadakis CA, Wong A, Genetos DC, Cheung WK, Borjesson DL, Ferraro GL, Galuppo LD, Leach JK, Owens SD Yellowley C.E (2010) Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am J Vet Res 71:1237–1245.
Tsubone T, Moran SL, Amadio PC, Zhao C, An KN (2004) Expression growth factors in canine flexor tendon after laceration in vivo. Ann Plast Surg 53:393–397
Vandenbergh A, Broeckx SY, Beerts C, Seys B, Zimmerman M, Verweire I, Suls M, Spaas J (2016) Tenogenically induced allogeneic mesenchymal stem cells for the treatment of proximal suspensory ligament desmitis in a horse. Front Vet Sci. doi:10.3389/fvets.2015.00049
Veronesi F, Torricelli P, Della Bella E, Pagani S, Fini M (2015) In vitro mutual interaction between tenocytes and adipose-derived mesenchymal stromal cells. Cytotherapy 17:215–223
Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE (2006) Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 15:1436–1449
Zhao B, Chen YG (2014) Regulation of TGF-β signal transduction. Scientifica (Cairo). doi:10.1155/2014/351095
Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P (2013) Manual Isolation of Adipose-Derived Stem Cells from Human Lipoaspirates J Vis Exp:26. doi:10.3791/50585
Acknowledgments
We thank Prof. Anthea Rowlerson (King’s College London, UK) for manuscript language revision. This work was supported by a grant from the University of Padova, Italy (prot. n. CPDA138242, PRAT 2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
JS and SB are employers of GST and inventors of several patents owned by the company.
None of the other authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Additional information
Marco Patruno and Jan Spaas Contributed equally.
Rights and permissions
About this article
Cite this article
Gomiero, C., Bertolutti, G., Martinello, T. et al. Tenogenic induction of equine mesenchymal stem cells by means of growth factors and low-level laser technology. Vet Res Commun 40, 39–48 (2016). https://doi.org/10.1007/s11259-016-9652-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-016-9652-y