Abstract
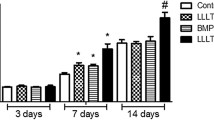
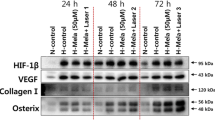
The aim of this study was to examine the effect of low-level laser therapy (LLLT) on the cell viability and the expression of hypoxia-inducible factor-1s (HIF-1s), bone morphogenic protein-2 (BMP-2), osteocalcin, type I collagen, transforming growth factor-β1 (TGF-β1), and Akt in hypoxic-cultured human osteoblasts. Human fetal osteoblast cells (cell line 1.19) were cultured under 1 % oxygen tension for 72 h. Cell cultures were divided into two groups. At the experimental side, low-level laser (808 nm, GaAlAs diode) was applied at 0, 24, and 48 h. After irradiation, each cell culture was incubated 24 h more under hypoxia. Total energy was 1.2, 2.4, and 3.6 J/cm2, respectively. Non-irradiated cultures served as controls. Comparisons between the two groups were analyzed by t test; a p value <0.05 was considered statistically significant. Hypoxia resulted in a decrease in the expression of type I collagen, osteocalcin, and TGF-β1 (p < 0.001, p < 0.001, and p < 0.01, respectively). Cell viability and BMP-2 expression were not decreased by hypoxic condition. On the other hand, LLLT on hypoxic-cultured osteoblast promoted the expression of BMP-2, osteocalcin, and TGF-β1 (p < 0.05, p < 0.01, and p < 0.001, respectively). Cell proliferation was also increased time-dependently. However, hypoxia decreased in type I collagen expression (p < 0.001), and LLLT did not affect type I collagen expression in hypoxic-cultured osteoblasts. Furthermore, LLLT inhibited HIF-1 and Akt expression in hypoxic conditioned osteoblasts. We concluded that LLLT induces the expression of BMP-2, osteocalcin, and TGF- β1 in 1 % hypoxic-cultured human osteoblasts.


Similar content being viewed by others
References
Vanderkooi JM, Erecinska M, Silver IA (1991) Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol 260:C1131–1150
Vaupel P, Schlenger K, Knoop C, Hockel M (1991) Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 51:3316–3322
Harrison JS, Rameshwar P, Chang V, Bandari P (2002) Oxygen saturation in the bone marrow of healthy volunteers. Blood 99:394
Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR (2006) Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res 312:1693–1702
Dinenno FA, Seals DR, DeSouza CA, Tanaka H (2001) Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol 531:573–579
Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE (1999) Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol 66:889–900
Spector JA, Mehrara BJ, Greenwald JA, Saadeh PB, Steinbrech DS, Bouletreau PJ, Smith LP, Longaker MT (2001) Osteoblast expression of vascular endothelial growth factor is modulated by the extracellular microenvironment. Am J Physiol Cell Physiol 280:C72–80
Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, Kraenkel N, Prezioso L, Emanueli C, Madeddu P (2010) Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol 30:498–508
Steinbrech DS, Mehrara BJ, Saadeh PB, Chin G, Dudziak ME, Gerrets RP, Gittes GK, Longaker MT (1999) Hypoxia regulates VEGF expression and cellular proliferation by osteoblasts in vitro. Plast Reconstr Surg 104:738–747
Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Gittes GK, Longaker MT (2000) VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. Am J Physiol Cell Physiol 278:C853–860
Tseng WP, Yang SN, Lai CH, Tang CH (2010) Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. J Cell Physiol 223:810–818
D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC (2006) Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 39:513–522
Lee CM, Genetos DC, You Z, Yellowley CE (2007) Hypoxia regulates PGE(2) release and EP1 receptor expression in osteoblastic cells. J Cell Physiol 212:182–188
Park JH, Park BH, Kim HK, Park TS, Baek HS (2002) Hypoxia decreases Runx2/Cbfa1 expression in human osteoblast-like cells. Mol Cell Endocrinol 192:197–203
Semenza GL (1998) Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 8:588–594
D'Angio CT, Finkelstein JN (2000) Oxygen regulation of gene expression: a study in opposites. Mol Genet Metab 71:371–380
Hwang JM, Weng YJ, Lin JA, Bau DT, Ko FY, Tsai FJ, Tsai CH, Wu CH, Lin PC, Huang CY, Kuo WW (2008) Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Mol Cell Biochem 311:179–187
Sharp FR, Bernaudin M (2004) HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5:437–448
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Renno AC, McDonnell PA, Parizotto NA, Laakso EL (2007) The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg 25:275–280
Kiyosaki T, Mitsui N, Suzuki N, Shimizu N (2010) Low-level laser therapy stimulates mineralization via increased Runx2 expression and ERK phosphorylation in osteoblasts. Photomed Laser Surg 28(Suppl 1):S167–172
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Li X, Cao X (2006) BMP signaling and skeletogenesis. Ann N Y Acad Sci 1068:26–40
Centrella M, McCarthy TL, Canalis E (1987) Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem 262:2869–2874
Centrella M, Massague J, Canalis E (1986) Human platelet-derived transforming growth factor-beta stimulates parameters of bone growth in fetal rat calvariae. Endocrinology 119:2306–2312
Lundy MW, Hendrix T, Wergedal JE, Baylink DJ (1991) Growth factor-induced proliferation of osteoblasts measured by bromodeoxyuridine immunocytochemistry. Growth Factors 4:257–264
Robey PG, Young MF, Flanders KC, Roche NS, Kondaiah P, Reddi AH, Termine JD, Sporn MB, Roberts AB (1987) Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol 105:457–463
Wrana JL, Maeno M, Hawrylyshyn B, Yao KL, Domenicucci C, Sodek J (1988) Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol 106:915–924
Mukherjee A, Rotwein P (2009) Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci 122:716–726
Choi YH, Jeong HM, Jin YH, Li H, Yeo CY, Lee KY (2011) Akt phosphorylates and regulates the osteogenic activity of Osterix. Biochem Biophys Res Commun 411:637–641
Kawamura N, Kugimiya F, Oshima Y, Ohba S, Ikeda T, Saito T, Shinoda Y, Kawasaki Y, Ogata N, Hoshi K, Akiyama T, Chen WS, Hay N, Tobe K, Kadowaki T, Azuma Y, Tanaka S, Nakamura K, Chung UI, Kawaguchi H (2007) Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS One 2:e1058
Warren SM, Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Bouletreau PJ, Longaker MT (2001) Hypoxia regulates osteoblast gene expression. J Surg Res 99:147–155
Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT (2004) Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem 279:40007–40016
Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K (2005) Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 26:3503–3509
Kim YD, Kim SS, Hwang DS, Kim SG, Kwon YH, Shin SH, Kim UK, Kim JR, Chung IK (2007) Effect of low-level laser treatment after installation of dental titanium implant-immunohistochemical study of RANKL, RANK, OPG: an experimental study in rats. Lasers Surg Med 39:441–450
Kim YD, Song WW, Kim SS, Kim GC, Hwang DS, Shin SH, Kim UK, Kim JR, Chung IK (2009) Expression of receptor activator of nuclear factor-kappaB ligand, receptor activator of nuclear factor-kappaB, and osteoprotegerin, following low-level laser treatment on deproteinized bovine bone graft in rats. Lasers Med Sci 24:577–584
Hirata S, Kitamura C, Fukushima H, Nakamichi I, Abiko Y, Terashita M, Jimi E (2010) Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J Cell Biochem 111:1445–1452
Fujimoto K, Kiyosaki T, Mitsui N, Mayahara K, Omasa S, Suzuki N, Shimizu N (2010) Low-intensity laser irradiation stimulates mineralization via increased BMPs in MC3T3-E1 cells. Lasers Surg Med 42:519–526
Houreld NN, Sekhejane PR, Abrahamse H (2010) Irradiation at 830 nm stimulates nitric oxide production and inhibits pro-inflammatory cytokines in diabetic wounded fibroblast cells. Lasers Surg Med 42:494–502
Zhang J, Xing D, Gao X (2008) Low-power laser irradiation activates Src tyrosine kinase through reactive oxygen species-mediated signaling pathway. J Cell Physiol 217:518–528
Kanichai M, Ferguson D, Prendergast PJ, Campbell VA (2008) Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol 216:708–715
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011–0026921).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Song WW and Dr. Pyo SJ equally contributed to this study as the first authors.
Rights and permissions
About this article
Cite this article
Pyo, SJ., Song, WW., Kim, IR. et al. Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-β1 in hypoxic-cultured human osteoblasts. Lasers Med Sci 28, 543–550 (2013). https://doi.org/10.1007/s10103-012-1109-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1109-0