Abstract
Rising temperatures due to climate change are expected to interplay with biological invasions, and may enhance the spread and growth of some alien species upon arrival in new areas. To successfully invade, a plant species needs to overcome multiple biological barriers. Among the crucial life stages, seed germination greatly contributes to the final species assembly of a plant community. Several studies have suggested that alien plant success is related to their high seed germination and longevity in the soil. Hence, our aim is to test if the germination potential of alien seeds present in the seed bank will be further enhanced by future warming in temperate dry grasslands, an ecosystem that is among those most prone to biological invasions. We designed a laboratory germination experiment at two temperatures (20 and 28 °C), to simulate an early or late heat wave in the growing season, using seeds from nine common grassland Asteraceae species, including native, archaeophyte and neophyte species. The test was performed on both single and mixed pools of these categories of species, using a full-factorial orthogonal design. The warmer germination temperature promoted neophyte success by increasing germination probability and germination speed, while negatively impacting these parameters in seeds of native species. The co-occurrence of native and archaeophyte seeds at the lower temperature limited the invasiveness of neophytes. These results provide important information on future management actions aimed at containing alien plant invasions, by improving our knowledge on the possible seed-bank response and interaction mechanisms of common species occurring in disturbed natural areas or restored sites.
Graphical abstract
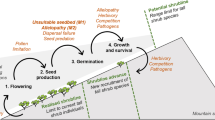
Summary of the experimental results. The colour of the flowers represent the status, divided as native (blue), neophyte (red) and archaeophyte (green). Each flower symbol represents the species pool for each plant category (i.e. NA = Buphthalmum salicifolium, Carlina vulgaris, Centaurea scabiosa; NE = Artemisia annua, Symphyotrichum novi-belgii, Senecio inaequidens; AR = Centaurea cyanus, Cichorium intybus, Tripleurospermum inodorum). The number of flowers represent the germination percentage of the various category assembly. In the columns are divided the various combination. From up to bottom the trend of germination percentage at 20 and 28 °C are shown.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global change is recognized as one of the major threats to biodiversity across most terrestrial biomes (Pressey et al. 2007). The last Intergovernmental Panel on Climate Change report showed an expected increase temperature of around 1.5 °C (IPCC 2022) in the next two decades, with significant consequences for species distribution (Parmesan and Yohe 2003). Climate change is also expected to increase extreme weather events in temperate zones, for example, increasing the frequency of heat waves which will affect plant growth and reproduction during the whole life cycle (Jolly et al. 2005; De Boeck et al. 2010; Perkins-Kirkpatrick and Lewis 2020).
Besides global warming, biological invasion is drastically reducing native biodiversity (Hulme 2009), causing a global homogenization of biological systems (McKinney and Lockwood 1999). Europe, alien species are commonly divided into two categories: archaeophytes (i.e. plants which arrived before 1492) and neophytes (i.e. plants which arrived after 1492), showing consistent distribution patterns and putatively similar mechanisms of spread (Chytrý et al. 2008). Nonetheless, archaeophytes are more frequent in agricultural areas (Chytrý et al. 2008; La Sorte and Pyšek 2009; Zając et al. 2009), while neophytes are more related to landscape disturbance caused by urbanization (Boscutti et al. 2022). Among the neophytes, invasive alien species (hereafter IAS) have shown their potential to: alter ecosystem processes (Root et al. 2003; Raizada et al. 2008); decrease native species abundance and richness via competition, predation, hybridization and indirect effects (Blackburn et al. 2004; Gaertner et al. 2009; Boscutti et al. 2020; Vitti et al. 2020); change native community structure (Hejda et al. 2009); alter nutrient cycling (Pellegrini et al. 2021a, b); impact landscape perception (Pejchar and Mooney 2009); and alter genetic diversity (Ellstrand and Schierenbeck 2000).
Climate change and biological invasion are two of the most studied issues in plant ecology, but their interplay has been less well studied. Indeed, rising temperatures are expected to enhance biological invasion by several mechanisms related to the phenotypic and genotypic plasticity of alien plants (Dukes and Mooney 1999; Jarnevich and Stohlgren 2009). In particular, higher temperatures might favour invasive alien species that are able to occupy new niches created by changes in local conditions (Bradley et al. 2010). IAS are usually well-adapted to cope with rising temperatures (Crooks and Soulé 1999), increasing their ability to replace more climate-sensitive native species (Byers 2002).
The success of establishment and spread of an introduced taxon and its invasive potential is related to its ability to overcome geographical and reproductive barriers (Blackburn et al. 2011), respond to disturbance (Geppert et al. 2021) and to spread in the landscape, mainly across human-made land uses (Pellegrini et al. 2021b; Boscutti et al. 2022). These abilities are often linked to plant traits. An ideal IAS has a short life cycle, self-compatibility, early and long flowering, high seed production and effective dispersal mechanism and high germination (Rejmanek and Richardson 1996; Pyšek and Richardson 2007). Among all traits, those related to seed germination play a crucial role in alien plant invasion (Lake and Leishman 2004), showing a potential interaction with land use change (Becker et al. 2016). In particular, rapid spread and colonization by an alien species from the original source populations is usually due to its germination success, speed and timing (Zhang et al. 2011). The faster the emergence of seedlings, the sooner the access to resources and space, enhancing the competitiveness of IAS against natives in the first stage of establishment (Wainwright et al. 2012; Gioria et al. 2018; Lami et al. 2021). Moreover, higher germination success increases the chance of establishment of alien propagules. This can lead to substantial plant and population growth before other species (Abraham et al. 2009). The positive effects of early germination on growth have been reported for several IAS (Abraham et al. 2009; Dickson et al. 2012; Vaughn and Young 2015) but less is known about the interaction at the seed level.
Asteraceae is counted as the largest eudicotyledonous angiosperm family with nearly 24,700 species (Stevens 2001; Christenhusz and Byng 2016). Among alien species, Asteraceae is one of the most represented families and one of the most prone to become invasive (Pyšek 1998). Indeed, this family possesses a number of advantageous features for the invasion process, e.g. high reproductive rate, specialized dispersal structures, diverse secondary metabolites providing protection from grazing, high levels of apomixis and self-compatibility (Heywood 1989; Pysek 1997; Hao et al. 2011), growth–defence/stress tolerance trade‐offs (Turner et al. 2014) and allelopathic substances (Vidotto et al. 2013; Kudryavtseva et al. 2020).
Among terrestrial ecosystems, temperate semi-natural dry grasslands are considered important hotspots of biodiversity (Wilson et al. 2012), but also one of the ecosystems most at risk of biological invasions (Boscutti et al. 2020). Species assembly in semi-natural grasslands represents the result of disturbances associated with cultivation and domestic grazing (Hobbs and Huenneke 1992). In recent times, this disturbance event has created the opportunity for alien species to invade those habitats (Boscutti et al. 2018). The susceptibility to invasion highlights the importance of understanding the invasion process and increasing the resistance of native grassland community species (Yannelli et al. 2017).
In the literature, several studies considered the plant germination response to temperature (Baskin and Baskin 1985; Iba 2002; Franklin 2009; Bewley and Black 2012), but few studies have considered this aspect in alien plants. Moreover, little is known about the plant-plant interactions occurring during the germination stage of alien and native plants (Schlau et al. 2023).
In this context, we designed a laboratory germination experiment aimed at elucidating the IAS success in future warming scenarios, as a driver of change in species composition of the grassland plant community. We selected common native, archaeophyte and neophyte grassland Asteraceae species, testing their germination in single- (i.e. seeds surrounded by species with the same alien status) and multi-category tests (factorial design; i.e. seeds surrounded by species with different alien status). We hypothesized that higher temperature would increase the likelihood of alien plants outperforming native plants during the germination stage by increasing their speed and probability of germination, both when sown alone or mixed with native plants. In particular, we postulated that there would be faster germination of neophytes at higher temperature and lower germination ability of native species, and that these trends are shaped by plant-plant interactions.
Material and methods
Plant species
This research was carried out on nine common dry grassland species belonging to the Asteraceae family, all occurring in the region of Friuli Venezia Giulia, North-East Italy. The species were selected according to their frequency and abundance in the local dry grassland. A brief description is included in the supplementary materials (Table S1). They were divided into three different plant categories according to Buccheri et al. (2019) and Poldini et al. (2002): native (hereafter NA), archaeophytes (hereafter AR) and neophytes (hereafter NE). The species chosen were: Buphthalmum salicifolium L. (NA), Carlina vulgaris L. (NA), Centaurea scabiosa L. (NA), Centaurea cyanus L. (AR), Cichorium intybus L. (AR), Tripleurospermum inodorum (L.) Sch.Bip (AR), Artemisia annua L. (NE), Symphyotrichum novi-belgii (L.) G.L.Nesom (NE) and Senecio inaequidens DC. (NE).
Seed collection
Seeds of Symphyotrichum novi-belgii and Senecio inaequidens were collected in January 2022, at the time of natural dispersal, in a disturbed dry grassland habitat in the neighbourhood of Udine, North-East Italy. All the other seeds were provided by SemeNostrum, a north-eastern Italian seed company member of the European Native Seed Producers Association. Seeds were harvested at the producer's nursery in summer 2021 from cultivated plants derived from germplasm of wild populations present in the Region. Harvested seeds were manually cleaned and kept for 2 months in paper bags at 15 °C and 15% relative humidity to ensure uniform seed drying and then stored in sealed vials in the same environment for approximately 3 months, when the germination experiment commenced. Preliminary germination tests were run on each species. The tests confirmed that the vitality was in a range between 70 and 94%, with a mean value of 88%. The seeds germinated without seed pre-treatment, with the exception of a brief sterilization with sodium hypochlorite (5% v/v, 10 min).
Experimental design
The pools of each plant category (i.e. NA, NE, AR) were represented by the three species selected (3species × 3 categories). Using the category pool species as experimental factor (i.e. NA, NE, AR), a full-factorial experiment was performed encompassing all the possible combinations between plant categories (Fig. 1, were each seed symbol represents a pool of 3 species of each category). In particular, the combination were: NA assembly (mix of the 3 native species), AR assembly (mix of the 3 archaeophyte species), NE assembly (mix of the 3 neophytes species), NA–AR assembly (mix of the 3 native and 3 archaeophyte species), NA-NE assembly (mix of the 3 native and 3 neophyte species), AR–NE assembly (mix of the 3 archaeophyte and 3 neophyte species), NA–AR–NE assembly (mix of the 3 native, 3 archaeophyte and 3 neophyte species). In this experiment, we did not consider the results about single separated species, focussing our aims on the interactions between species of the same or different status. Seed pattern distribution on each replicate was assigned with the aim of testing all the different interactions. The seed allocation was designed randomly, but consistently throughout the replicates (Fig. S1). More specifically, the seeds were placed on the plate in such a way that each species was surrounded at least once by every other species present. A paper grid was placed at the base of the plate to keep track of the observed species. Seeds were not clean out of their pappuses. Each seed was made recognisable following a distribution scheme of each Petri dish. The seeds were evenly distributed in the Petri dish, with an average distance of 10 mm between each seed at the start of the experiment.
Experimental design. From left to right: species chosen divided by status; Venn-diagram showing the seven assembly combinations based on status; and the two temperatures tested. Each seed symbol represents the species pool for each plant category (i.e. NA = Buphthalmum salicifolium, Carlina vulgaris, Centaurea scabiosa; NE = Artemisia annua, Symphyotrichum novi-belgii, Senecio inaequidens; AR = Centaurea cyanus, Cichorium intybus, Tripleurospermum inodorum)
Each combination was replicated five times under two temperature regimes (n = 70; 7 species assembly combinations × 2 temperatures × 5 replicates). The two temperatures were 20 and 28 °C, to simulate a heatwave due to climate change during the early germination stages. The choice of the high temperature treatment was done to mimic the effect of a late or early heat wave during the growing season, according to the seasonal temperature trends of the region.
Germination assays
Germination assays were performed using six seeds of each species per replicate, sown in Petri dishes of 90, 120 or 150 mm \(\emptyset\)\(,\) for single, double or triple species category assemblies, respectively. Seeds were placed on 0.8% agar–water solution. The Petri dishes were randomly arranged in thermostatically controlled growth cabinets (20 and 28 °C), equipped with cool white fluorescent tubes (Osram FL 40 SS W/37) providing 14 h light day−1, simulating the photoperiod that occurs at this region latitudes during late spring. All Petri dishes were wrapped with Parafilm M® to prevent moisture loss. The dishes were monitored daily and RGB images were captured by digital camera (Panasonic, model Lumix GH5) with a high resolution macro lens. Based on the captured images, seeds with visible radicle protrusion were counted as germinated. Germinated seeds were not removed after germination but remained in the Petri dish as well as not germinated seeds. The final germination percentage was scored after an incubation period of at least 20 d, until no more germinated seeds were recorded over 3 consecutive days. At the end of each germination test, non-germinated seeds were cut-tested, confirming that they were viable.
Statistical analyses
The germination records were analysed considering three main species assembly groups: single category assembly (i.e. NA, NE and AR), double category assembly (i.e. NA–NE, NA–AR and AR–NE) and triple category assembly (i.e. NA–AR–NE). The germination behaviour of each status assembly was analysed separately by nonlinear regression. The model choice and parameter estimation were based on time-to-event analysis (i.e. for the model it is necessary to consider viable seeds that have not germinated/emerged at the end of an experiment; Onofri et al. 2010, 2022), also providing inferences on germination events that did not occur at the specific time of evaluation, but during the interval between evaluations (Ritz et al. 2013). The model is log-logistic and the formula includes 3 germination parameters as follows:
where F(t) is the fraction of germinated seeds between time interval t, d is the parameter referring to the total germination (i.e. the higher asymptote of the function), b is the slope of the curve at the inflection point (i.e. usually described as seed uniformity) and e is the time needed to reach 50% of the total germination (usually known as T50), here used as a measure of germination speed.
All the statistical analyses were performed in R environment (R Core Team 2022). The dataset was prepared for analyses using the function “mel_te” (i.e. reshaping time-to-event datasets) of the “drcte” R package (Onofri 2022), an implementation of the “drc” package (Ritz et al. 2015). The model was performed using the “drmte” function (i.e. fitting time-to-event models for seed science; Onofri 2022). The difference between b, d and e parameters between different community assemblies and the two temperatures were tested with the function “compCDF” (i.e. compare time-to-event curves, comparing the difference in each model; Benaglia et al. 2010). The difference was considered statistically significant when the p value was < 0.05.
The main responses of the seed community were summarized by Richards diagrams showing the trends in the parameters d (i.e. germination percentage), e (i.e. T50) and b (i.e. seed uniformity) in the interactions between the status and treatments.
Results
The results are presented in three sections considering the (i) germination probability (i.e. d parameter), (ii) speed (i.e. e parameter) and (iii) uniformity (i.e. b parameter). Within each section we first show the status response, using the nonlinear model trends (Figs. 2, 3, 4), and consequently the overall plant community response, using the Richards diagrams (Fig. 5).
Seed germination progress for single category assemblies of species: natives (blue), archaeophytes (green) and neophytes (red). The circles represent the observed germination proportion; the lines (cumulative probability of germination) show the results of fitting Eq. (1) to the data. The empty dots and dashed lines represent the 20 °C treatment; the full dots and lines represent the 28 °C treatment. Inset table shows the results of comparing value of d or e parameters between the various species categories. p value is expressed as follows: ns (p value > 0.1), •(0.1 < p value < 0.05), *(0.05 < p value < 0.01), **(0.01 < p value < 0.001), ***(p value < 0.001)
Seed germination curve of double category assembly. The colours represent categories as follows: blue (natives), green (archaeophytes), red (neophytes). The empty dots and dashed lines represent the 20 °C treatment. The full dots and lines represent the 28 °C treatment. Inset table shows the results of comparing value of d or e parameters between the various species categories. p value is expressed as follows: ns (p value > 0.1), •(0.1 < p value < 0.05), *(0.05 < p value < 0.01), **(0.01 < p value < 0.001), ***(p value < 0.001)
Seed germination curve of triple category assembly of species (i.e. neophytes, archaeophytes and natives). The colours represent categories as follows: blue (natives), green (archaeophytes), red (neophytes). The empty dots and dashed lines represent the 20 °C treatment. The full dots and lines represent the 28 °C treatment. Inset table shows the results of comparing value of d or e parameters between the various species categories. p value is expressed as follows: ns (p value > 0.1), •(0.1 < p value < 0.05), *(0.05 < p value < 0.01), **(0.01 < p value < 0.001), ***(p value < 0.001)
Richards diagrams showing the behaviour of the various species assemblies at 20 and 28 °C. The parameters shown are the d, e and b parameters derived from the germination progress curves (Onofri et al. 2010, 2022). They represent respectively: the higher asymptote (i.e. the maximum proportion of germinated seeds), the median germination time (i.e. T50) and the slope at the inflection point (i.e. seed uniformity). The ‘number of category assembly’ refers to how many species status categories were evaluated. The colours describe status as follows: blue (natives), green (archaeophytes), red (neophytes)
Effects of temperature on alien and native plant germination probability
Temperature did not significantly affect the germination probability of NA, NE or AR species in their single assemblies. The highest germination probability was achieved by NE at 28 °C in the single category assembly (i.e. neophytes surrounded by only neophytes) with 83.2% seed germination probability. (Fig. 2). The lowest was found in AR at 20 °C in the single category assembly, with 44.4% seed germination probability. NE germination probability was always higher than AR, but only reached higher germination probability compared with NA at the higher temperature in the single assembly.
Differences in germination probability were found when comparing NA and NE at 20 °C in a double category assembly (Fig. 3). The germination probability of NE seeds in this NA–NE assembly was also significantly different between 20 and 28 °C (p value < 0.05). The seeds of AR species displayed no significant difference in the double category assembly at different temperatures. A lower germination was noticed for NA species seeds at 28 °C compared with 20 °C in the NA–AR assembly. NE exhibited higher germination probability compared with AR in the double category assembly, regardless of the temperature.
In the triple category assembly, at 20 °C, NA species seeds exhibited the highest germination probability (80%), with a significant difference between each of the other species categories or temperature considered (Fig. 4). The high temperature did not affect NE seed germination (p value > 0.05), but it decreased the germination probability of NA seeds. In this triple category assembly, NE seeds had a significant higher germination probability than NA seeds at 28 °C (p value = 0.087, 2-way ANOVA, Fig. 4).
Overall, the plant community seems to have consistent germination probability at 20 °C between the various category assemblies, except for the NA with AR (Fig. 5a). On the contrary, at 28 °C we found a general decrease in the germination probability of the plant community, compared with the single category assembly, mainly due to lower germination of NA seeds (Fig. 5b). As found at 20 °C, at 28 °C the plant community showed a decrease in germination probability when NA seeds are in proximity with AR seeds.
Effects of temperature on alien and native plant germination speed
Germination speed varied between 20 and 28 °C within the various status assemblies (Figs. 2, 3, 4). In particular, AR and NE seeds germinated faster at the highest temperature. In the single category assembly, NA seeds were the slowest to germinate while AR seeds were the fastest (Fig. 2). NA seeds showed no significant differences in germination speed between the different temperatures in the single assembly.
No difference was found in germination speed between NA and NE seeds at 20 °C in the double category assembly (Fig. 3). Moreover, no difference due to increased temperature was observed for NA seeds in double category assembly. NE seed germination at 28 °C was faster than NE germination at 20 °C and NA germination at 28 °C. In the NA–AR assembly, temperature appears to have a significant role in increasing the speed of germination, both for NA and AR seeds. Similar results were found in the NE–AR assembly, where germination was faster at 28 °C than at 20 °C.
Remarkably, in the triple category assembly, NA seeds at 20 °C were the slowest to germinate (Fig. 4). No differences were found between the speed of germination of NA seeds at 28 °C or of NE seeds at either 20 or 28 °C. AR were the fastest in the triple category assembly, with 28 °C AR faster than 20 °C. We also found that temperature increased the speed of germination of NE seeds.
Overall, the plant communities respond differently to the increase in temperature (Fig. 5). In particular, at 20 °C, seed performance in the double assembly communities seems to be quite consistent on seed germination speed in the single assembly, in particular the one involving NA and NE. Interestingly, we found that for either the double or triple category assemblies, temperature enhanced the speed of germination of the overall plant communities. In particular, at 20 °C the mean T50 of the plant community is around 4.3 days while at 28 °C it is less than 3 days (Fig. 5c, d).
Effects of temperature on alien and native plant germination uniformity
The b parameter showed consistent results across the experiment (Tab. S5). In particular, in the single category assembly, there were no differences between treatment and alien status. In the double category assembly, we found that temperature tended to increase the germination uniformity (i.e. lower b value). In particular, this was true in NA–AR for both NA and AR seeds (p value NA = 0.0015, p value AR = 0.0006, compared with the b-values at 20 °C), in NA-NE the increase in seed uniformity was for NA seeds (p value = 0.025) and in AR–NE, the increase was found for NE seeds (p value = 0.0035). In the triple category assembly, NE seeds had higher germination uniformity at 28 °C (p value = 0.0013).
The overall community response (Fig. 5e, f) displayed slightly differences between the various interactions of category assembly, with the AR-NA less uniform that NA-NE at 20 °C. Interestingly, we found that the germination uniformity of the plant community was higher (i.e. lower value of b) at 28 °C.
Discussion
Increasing temperatures due to climate change and biological invasion are among the top most threats to biodiversity, often interacting with other environmental factors in altering the ecosystem equilibrium (Bellard et al. 2022; Jaureguiberry et al. 2022). Our study uniquely evidenced that the seed interactions between different alien and native plants significantly affect their germination under a simulated heatwave during germination stage, which are expected to be more intense and frequent due to climate change (Perkins-Kirkpatrick and Lewis 2020). While we acknowledge that these findings may not be entirely reflective of the whole plant community, we consider the responses exhibited by some of the most common species to be somewhat insightful, highlighting the need for future studies. In particular, we found temperature to be a key factor for seed germination of the community created by the pools of study species, shaping the uniformity, the timing (i.e. speed) and abundance (i.e. probability) of seedling establishment (Fig. 5). Moreover, the temperature effect depended on the plant alien status (i.e. natives, neophytes, archaeophytes) and their interaction (i.e. when comparing seeds germinating surrounded by species of the same status to seeds germinating in different assembly status combinations). Higher temperature seems to be detrimental for the germination success of NA species when surrounded by NE and AR, while NE germination potential is not influenced as much by a warmer climate. Temperature also enhanced the germination speed of NE and AR species. Interestingly, at low temperature, NA species exhibited higher germination success compared with NE and AR species (Figs. 3, 4), in the double and triple category assemblies, suggesting their potential capacity to curb the studied alien species establishment at low temperatures. We also found that temperature increases germination uniformity of all species, independent of the seed mixture.
Our data suggest that a future warmer temperature might undermine the studied native Asteraceae species germination only when surrounded by alien species, favouring the success of alien plants. In the light of the increased temperature due to climate change and related heat wave event intensification (Meehl and Tebaldi 2004; Perkins-Kirkpatrick and Lewis 2020), we believe these results may help in the understanding of future plant community assembly.
Effects of temperature and species interaction on seed germination parameters
Seeds of NA species did not have different germination probabilities under the tested temperatures when seeds were surrounded by other native species, similar to reports for other Asteraceae species (Hou et al. 2014). However, the germination of NA seeds in the triple category assembly (i.e. close to NE and AR seeds) was affected by temperature: low temperature enhanced the germination success of NA seeds while no difference was observed in the germination of NE or AR seeds in the same assembly depending on temperature (Fig. 4). Hence, warmer temperatures may significantly shape the final plant community assembly, by reducing the germination success of native plant species. The implication of this result is that, at low temperature, seeds of native Asteraceae species appear to be more likely to germinate, thus leading to a larger number of individuals compared with NE or AR species. This seems to be an important result, given that the number of individuals from a population can act as a barrier to biological invasion by reducing the ecological space for alien plants (Oduor 2013). It is also consistent with the results of Levine et al. (2004) where the biotic resistance displayed by the native community seldom empowers them to repel invasions, opting instead to lower the population of invasive species post-establishment, similarly to what occurs in forest fragments (Trotta et al. 2023). In particular, the relative size of the native population studied may affect the way the native responds to alien species competition due to their biomass (Dawson et al. 2011), phenotypic plasticity, genetic drift or genetic diversity (Strauss et al. 2006; Leger and Espeland 2010).
Interestingly, we found that temperature did not affect the germination probability of NE seeds in both the pure NE assembly and in mixtures with seeds of NA or AR species (Figs. 2, 3). Moreover, the lack of major temperature effects on germination probability of NE seeds is consistent with previous results (Jeffery et al. 1988; López-García and Maillet 2005; Hou et al. 2014), highlighting that temperature may not reduce the invasiveness of neophytes species acting on seed germination, due to their better performance at this critical stage (seedling establishment) of the plant life cycle. This can probably be explained by the fact that invasive species (neophytes in our case) are more wide-temperature tolerant, and this trait has led them to become invasive (Thuiller et al. 2007), also in temperate dry grasslands. Indeed, our results show that while increasing temperature decreases the probability of germination for NA species, there was no impact on the germination of NE species in the warmer environment. In fact, low temperatures are a limiting factor for the spread of alien plant propagules (Alexander et al. 2011; Marini et al. 2013; Lembrechts et al. 2016). Contrary to our expectations, AR species had lower germination potential compared with the other species, in particular at 20 °C in the single category assembly (Fig. 2).
The speed of seed germination (i.e. expressed as the e parameters of our model) is also crucial to plant community assembly dynamics, being the timing at which plants make their first access to environmental resource (Gioria et al. 2018). Our results suggest that at low temperature, in a single status community, NE and NA seeds reach T50 at the same time, while at 28 °C NE seeds were faster to germinate than NA seeds. Conversely, NE seeds germinated significantly faster than NA seeds when sown in a mixture with the other status at 20 °C. At this lower temperature, NE seeds appear to germinate earlier than natives, gaining the benefit of access to environmental resources before competitors (Gioria and Pyšek 2017). This is consistent with studies showing that a germination advantage or priority is a key factor facilitating plant invasions in many ecosystems, especially invasions by annual plants (Godoy et al. 2009; Abraham et al. 2009; Wainwright and Cleland 2013). At higher temperature, in contrast to our expectations, NE and NA seeds had similar germination speeds. Moreover, NA seeds at 28 °C in the assembly with NE and AR germinated faster compared with the NA seeds at low temperature in the same category assembly (Fig. 3). This increase is a recognized phenomenon that occurs during seed germination at high temperature (Roberts 1988), where the rate of seed metabolism is accelerated by moderately warmer temperatures (i.e. too high temperature will inhibit the germination as well). This has important implications on seed germination dynamics under the effect of global change, as already shown in other studies (Cochrane et al. 2011; Newton et al. 2020).
Archaeophytes appear to be the species with the earliest germination compared to the other species, with an increase in speed at high temperature (Figs. 2, 3, 4). It has been demonstrated that early-germinating species may have several benefits, such as size-asymmetric competition (Abraham et al. 2009), resource pre-emption (Ross and Harper 1972) and detrimental effects on the establishment and diversity of later-germinating species (Cabin et al. 2000; Rice and Dyer 2001). The timing of germination determines the post-germination conditions experienced by seedlings (Donohue 2003, 2005), including the competitive environment (Forbis 2010) and can strongly affect the fitness, growth and survival of a species in a community (Donohue 2005; Verdú and Traveset 2005), and thus its invasiveness or resistance to invasion.
Effects on the final species assembly
Our results suggest that temperature is a key factor that can modify the future assembly of plant communities, as abiotic filter acting at the germination stage. In particular, it could be possible to make some speculations on the way the plant community of dry grassland can be affected by climate change. We are aware that these findings might not be representative of the whole plant community, but we believe that response shown by some of the most common species is somewhat revealing and future studied are needed. Based on the results of our species, we can hypothesize that the response of the plant community will depend on three principal factors: (i) number of individuals, (ii) increasing temperatures and (iii) interaction among different types of species, specifically NA and AR species versus NE species.
NE seeds exhibit a higher germination percentage and speed at 28 °C when not surrounded by seeds of other status, compare with NA seeds. Invading species may need to easily access resources such as light, nutrients and water, and will therefore have greater success in invading a community if it does not encounter intense competition for these resources from resident species (Davis et al. 2000). Thus, future climate conditions will probably exacerbate the problem of plant invasion in the highly disturbed environment of already-degraded temperate grasslands, where NE species are commonly surrounded by other NE species. Populations of NA species, reduced by NE plants, appear to be less able to compete with IAS (Holle and Simberloff 2005).
For example, warmer temperatures caused by early season heat waves, will accelerate seed germination speed. The overall plant community will grow faster at warmer temperature, with no significant difference depending on species status in terms of germination potential. However, the difference between NA and NE species was almost significant (p value = 0.087), with the NE species seeds reaching higher germination probability (Fig. 2). Once again, this provides evidence that increasing temperature might increase the invasiveness potential of NE species, in terms of germination speed. We also want to underline that our experiment focussed on a temperature range compatible with seed germination, conscious that extreme heat might have negative effects on any of NA, AR or NE.
Finally, at low temperature, the presence of NA and AR seeds curbed the success of neophytes, with an increase in germination potential of NA, significantly higher than the competitors. We believe that the faster germination rate of the AR seeds hinders the germination of NE seeds, through competition for resources (Gioria and Pyšek 2017) or allelopathic effects (Chen et al. 2017). Indeed, there is increasing interest in possible allelopathic effects exerted by plants against IAS. Many native species produce allelochemicals that may function as a weapon against IAS, and thus strengthen the resistance of native species communities to exotic invasion (Zhao et al. 2008; Yu et al. 2011; Christina et al. 2015).
The leached of organic substances can hinder or facilitate the germination of neighbouring seeds (Renne et al. 2014), thus leading to the importance of understanding how the seeds interact in the seed bank. Choosing the right timing of germination appears to be crucial for the seedling development (Cohen 1967). Early germination can indeed be considered adaptive in many plant species. This phenomenon is often driven by a variety of ecological and evolutionary factors, and one of the key advantages is the potential for fast-emerging seedlings to gain a competitive edge over slower-emerging ones (Tielbörger and Prasse 2009; Gioria and Pyšek 2017).
Although NA are slower to germinate in the triple category assembly, they appear to be more competitive in terms of germination potential. Thus, the presence of a well-formed and biodiverse plant community appears to be one of the key factors to preserve the high value of dry temperate grasslands.
Seed germination and seedling emergence may also be strongly influenced by other factors not considered in this experiment, such as maternal effects (Roach and Wulff 1987) or seed density (Laterra and Bazzalo 1999). All the Asteraceae species considered produce a large number of seeds each reproductive season, but with some variation depending on the species, so their density in the seed bank may be variable, which is relevant information to consider in future research (Guido et al. 2017).
We demonstrated that some common native and archaeophytes grassland’ species not only have the potential to delay NE plant germination, but also to decrease its final germination percentage. These results may reveal potential solutions for restoring invaded areas by reintroducing the native and archaeophyte studied seeds to the seed bank, as delays in germination and growth can have important implications for preventing NE colonization in dry grasslands. The timing of this reintroduction appears to be critical. Reintroduction plans should focus on early-spring, when heat events are less likely, at least under current climate projections (IPCC 2022).
Data Availability
The dataset is fully available at Mendeley repository, https://doi.org/10.17632/5hx36chvbf.1
References
Abraham JK, Corbin JD, D’Antonio CM (2009) California native and exotic perennial grasses differ in their response to soil nitrogen, exotic annual grass density, and order of emergence. Plant Ecol 201:445–456. https://doi.org/10.1007/s11258-008-9467-1
Alexander JM, Kueffer C, Daehler CC, Edwards PJ, Pauchard A, Seipel T, Consortium M, Arévalo J, Cavieres L, Dietz H (2011) Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc Natl Acad Sci 108:656–661
Baskin JM, Baskin CC (1985) The annual dormancy cycle in buried weed seeds: a continuum. Bioscience 35:492–498
Becker M, Alvarez M, Heller G, Leparmarai P, Maina D, Malombe I, Bollig M, Vehrs H (2016) Land-use changes and the invasion dynamics of shrubs in Baringo. J East Afr Stud 10:111–129. https://doi.org/10.1080/17531055.2016.1138664
Bellard C, Marino C, Courchamp F (2022) Ranking threats to biodiversity and why it doesn’t matter. Nat Commun 13:2616. https://doi.org/10.1038/s41467-022-30339-y
Benaglia T, Chauveau D, Hunter DR, Young DS (2010) mixtools: an R package for analyzing mixture models. J Stat Softw 32:1–29. https://doi.org/10.18637/jss.v032.i06
Bewley JD, Black M (2012) Physiology and biochemistry of seeds in relation to germination: volume 2: viability, dormancy, and environmental control. Springer Science & Business Media, Berlin
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on oceanic islands. Science 305:1955–1958. https://doi.org/10.1126/science.1101617
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JRU, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339. https://doi.org/10.1016/j.tree.2011.03.023
Boscutti F, Sigura M, Simone SD, Marini L (2018) Exotic plant invasion in agricultural landscapes: a matter of dispersal mode and disturbance intensity. Appl Veg Sci 21:250–257. https://doi.org/10.1111/avsc.12351
Boscutti F, Pellegrini E, Casolo V, de Nobili M, Buccheri M, Alberti G (2020) Cascading effects from plant to soil elucidate how the invasive Amorpha fruticosa L. impacts dry grasslands. J Veg Sci 31:667–677
Boscutti F, Lami F, Pellegrini E, Buccheri M, Busato F, Martini F, Sibella R, Sigura M, Marini L (2022) Urban sprawl facilitates invasions of exotic plants across multiple spatial scales. Biol Invasions 24:1497–1510. https://doi.org/10.1007/s10530-022-02733-6
Bradley BA, Blumenthal DM, Wilcove DS, Ziska LH (2010) Predicting plant invasions in an era of global change. Trends Ecol Evol 25:310–318. https://doi.org/10.1016/j.tree.2009.12.003
Buccheri M, Boscutti F, Pellegrini E, Martini F (2019) La flora aliena nel Friuli Venezia Giulia. Gortania 40:7–78
Byers JE (2002) Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 97:449–458. https://doi.org/10.1034/j.1600-0706.2002.970316.x
Cabin RJ, Marshall DL, Mitchell RJ (2000) The demographic role of soil seed banks. II. Investigations of the fate of experimental seeds of the desert mustard Lesquerella fendleri. J Ecol 88:293–302
Chen B-M, Liao H-X, Chen W-B, Wei H-J, Peng S-L (2017) Role of allelopathy in plant invasion and control of invasive plants. Allelopathy J 41:155–166
Christenhusz MJ, Byng JW (2016) The number of known plants species in the world and its annual increase. Phytotaxa 261:201–217
Christina M, Rouifed S, Puijalon S, Vallier F, Meiffren G, Bellvert F, Piola F (2015) Allelopathic effect of a native species on a major plant invader in Europe. Sci Nat 102:1–8
Chytrý M, Jarošík V, Pyšek P, Hájek O, Knollová I, Tichý L, Danihelka J (2008) Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 89:1541–1553
Cochrane A, Daws MI, Hay FR (2011) Seed-based approach for identifying flora at risk from climate warming. Austral Ecol 36:923–935. https://doi.org/10.1111/j.1442-9993.2010.02211.x
Cohen D (1967) Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. J Theor Biol 16:1–14. https://doi.org/10.1016/0022-5193(67)90050-1
Crooks JA, Soulé ME, Sandlund OT (1999) Lag times in population explosions of invasive species: causes and implications. Invasive Species Biodiv Manag 24:103–125
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88(3):528–534. https://doi.org/10.1046/j.1365-2745.2000.00473.x
Dawson W, Fischer M, van Kleunen M (2011) The maximum relative growth rate of common UK plant species is positively associated with their global invasiveness. Glob Ecol Biogeogr 20:299–306
De Boeck HJ, Dreesen FE, Janssens IA, Nijs I (2010) Climatic characteristics of heat waves and their simulation in plant experiments. Glob Change Biol 16:1992–2000. https://doi.org/10.1111/j.1365-2486.2009.02049.x
Dickson TL, Hopwood JL, Wilsey BJ (2012) Do priority effects benefit invasive plants more than native plants? An experiment with six grassland species. Biol Invasions 14:2617–2624. https://doi.org/10.1007/s10530-012-0257-2
Donohue K (2003) Setting the stage: phenotypic plasticity as habitat selection. Int J Plant Sci 164:S79–S92
Donohue K (2005) Niche construction through phenological plasticity: life history dynamics and ecological consequences. New Phytol 166:83–92
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139. https://doi.org/10.1016/S0169-5347(98)01554-7
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci 97:7043–7050. https://doi.org/10.1073/pnas.97.13.7043
Forbis TA (2010) Germination phenology of some Great Basin native annual forb species. Plant Species Biol 25:221–230
Franklin KA (2009) Light and temperature signal crosstalk in plant development. Curr Opin Plant Biol 12:63–68. https://doi.org/10.1016/j.pbi.2008.09.007
Gaertner M, Den Breeyen A, Hui C, Richardson DM (2009) Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: a meta-analysis. Prog Phys Geogr 33:319–338. https://doi.org/10.1177/0309133309341607
Geppert C, Boscutti F, La Bella G, De Marchi V, Corcos D, Filippi A, Marini L (2021) Contrasting response of native and non-native plants to disturbance and herbivory in mountain environments. J Biogeogr 48:1594–1605. https://doi.org/10.1111/jbi.14097
Gioria M, Pyšek P (2017) Early bird catches the worm: germination as a critical step in plant invasion. Biol Invasions 19:1055–1080. https://doi.org/10.1007/s10530-016-1349-1
Gioria M, Pyšek P, Osborne BA (2018) Timing is everything: does early and late germination favor invasions by herbaceous alien plants? J Plant Ecol 11:4–16. https://doi.org/10.1093/jpe/rtw105
Godoy O, Castro-Díez P, Valladares F, Costa-Tenorio M (2009) Different flowering phenology of alien invasive species in Spain: evidence for the use of an empty temporal niche? Plant Biol 11:803–811
Guido A, Hoss D, Pillar VD (2017) Exploring seed to seed effects for understanding invasive species success. Perspect Ecol Conserv 15:234–238. https://doi.org/10.1016/j.pecon.2017.07.006
Hao JH, Qiang S, Chrobock T, van Kleunen M, Liu QQ (2011) A test of Baker’s law: breeding systems of invasive species of Asteraceae in China. Biol Invasions 13:571–580
Hejda M, Pyšek P, Jarošík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403. https://doi.org/10.1111/j.1365-2745.2009.01480.x
Heywood VH (1989) Patterns, extents and modes of invasions by terrestrial plants. Biol Invasions Glob Perspect 31–60
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity, and invasion: implications for conservation. Conserv Biol 6:324–337
Holle BV, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212–3218. https://doi.org/10.1890/05-0427
Hou Q-Q, Chen B-M, Peng S-L, Chen L-Y (2014) Effects of extreme temperature on seedling establishment of nonnative invasive plants. Biol Invasions 16:2049–2061. https://doi.org/10.1007/s10530-014-0647-8
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18. https://doi.org/10.1111/j.1365-2664.2008.01600.x
Iba K (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53:225–245. https://doi.org/10.1146/annurev.arplant.53.100201.160729
IPCC (2022) Summary for policymakers [H.-O. Pörtner, D.C. Roberts, E.S. Poloczanska, K. Mintenbeck, M. Tignor, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem (eds.)]. In: Climate change 2022: impacts, adaptation and vulnerability. Contribution of Working Group II to the sixth assessment report of the Intergovernmental Panel on Climate Change [H.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (eds.)]. Cambridge University Press, Cambridge and New York, pp 3–33. https://doi.org/10.1017/9781009325844.001
Jarnevich CS, Stohlgren TJ (2009) Near term climate projections for invasive species distributions. Biol Invasions 11:1373–1379. https://doi.org/10.1007/s10530-008-9345-8
Jaureguiberry P, Titeux N, Wiemers M, Bowler DE, Coscieme L, Golden AS, Guerra CA, Jacob U, Takahashi Y, Settele J (2022) The direct drivers of recent global anthropogenic biodiversity loss. Sci Adv 8:eabm9982
Jeffery DJ, Holmes PM, Rebelo AG (1988) Effects of dry heat on seed germination in selected indigenous and alien legume species in South Africa. S Afr J Bot 54:28–34. https://doi.org/10.1016/S0254-6299(16)31358-8
Jolly WM, Dobbertin M, Zimmermann NE, Reichstein M (2005) Divergent vegetation growth responses to the 2003 heat wave in the Swiss Alps. Geophys Res Lett. https://doi.org/10.1029/2005GL023252
Kudryavtseva EI, YuKir V, Viting KB, Kozyreva AM, Nefedova AD, Petrash EG, Stukalov AS, Sheynova AD, Reshetnikova NM (2020) The settlement of Erigeron annuus (L.) Pers. and analysis of the reasons for reproductive success. Russ J Biol Invasions 11:225–237. https://doi.org/10.1134/S2075111720030054
La Sorte FA, Pyšek P (2009) Extra-regional residence time as a correlate of plant invasiveness: European archaeophytes in North America. Ecology 90:2589–2597
Lake JC, Leishman MR (2004) Invasion success of exotic plants in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biol Conserv 117:215–226. https://doi.org/10.1016/S0006-3207(03)00294-5
Lami F, Vitti S, Marini L, Pellegrini E, Casolo V, Trotta G, Sigura M, Boscutti F (2021) Habitat type and community age as barriers to alien plant invasions in coastal species-habitat networks. Ecol Ind 133:108450. https://doi.org/10.1016/j.ecolind.2021.108450
Laterra P, Bazzalo ME (1999) Seed-to-seed allelopathic effects between two invaders of burned Pampa grasslands. Weed Res 39:297–308
Leger EA, Espeland EK (2010) PERSPECTIVE: coevolution between native and invasive plant competitors: implications for invasive species management. Evol Appl 3:169–178. https://doi.org/10.1111/j.1752-4571.2009.00105.x
Lembrechts JJ, Pauchard A, Lenoir J, Nuñez MA, Geron C, Ven A, Bravo-Monasterio P, Teneb E, Nijs I, Milbau A (2016) Disturbance is the key to plant invasions in cold environments. Proc Natl Acad Sci 113:14061–14066
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989. https://doi.org/10.1111/j.1461-0248.2004.00657.x
López-García MC, Maillet J (2005) Biological characteristics of an invasive south African species. Biol Invasions 7:181–194. https://doi.org/10.1007/s10530-004-8978-5
Marini L, Bertolli A, Bona E, Federici G, Martini F, Prosser F, Bommarco R (2013) Beta-diversity patterns elucidate mechanisms of alien plant invasion in mountains. Glob Ecol Biogeogr 22:450–460
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453. https://doi.org/10.1016/S0169-5347(99)01679-1
Meehl GA, Tebaldi C (2004) More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305:994–997
Newton RJ, Hay FR, Ellis RH (2020) Temporal patterns of seed germination in early spring-flowering temperate woodland geophytes are modified by warming. Ann Bot 125:1013–1023. https://doi.org/10.1093/aob/mcaa025
Oduor AMO (2013) Evolutionary responses of native plant species to invasive plants: a review. New Phytol 200:986–992. https://doi.org/10.1111/nph.12429
Onofri A (2022) drcte: Statistical Approaches for Time-to-Event Data in Agriculture. R package version 1.0.30
Onofri A, Gresta F, Tei F (2010) A new method for the analysis of germination and emergence data of weed species. Weed Res 50:187–198. https://doi.org/10.1111/j.1365-3180.2010.00776.x
Onofri A, Mesgaran MB, Ritz C (2022) A unified framework for the analysis of germination, emergence, and other time-to-event data in weed science. Weed Sci 70:259–271. https://doi.org/10.1017/wsc.2022.8
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. https://doi.org/10.1038/nature01286
Pejchar L, Mooney HA (2009) Invasive species, ecosystem services and human well-being. Trends Ecol Evol 24:497–504
Pellegrini E, Boscutti F, Alberti G, Casolo V, Contin M, De Nobili M (2021a) Stand age, degree of encroachment and soil characteristics modulate changes of C and N cycles in dry grassland soils invaded by the N2-fixing shrub Amorpha fruticosa. Sci Total Environ 792:148295. https://doi.org/10.1016/j.scitotenv.2021.148295
Pellegrini E, Buccheri M, Martini F, Boscutti F (2021b) Agricultural land use curbs exotic invasion but sustains native plant diversity at intermediate levels. Sci Rep 11:8385. https://doi.org/10.1038/s41598-021-87806-7
Perkins-Kirkpatrick SE, Lewis SC (2020) Increasing trends in regional heatwaves. Nat Commun 11:3357. https://doi.org/10.1038/s41467-020-16970-7
Poldini L, Vidali M, Oriolo G, Jogan N (2002) Nuovo Atlante corologico delle piante vascolari nel Friuli Venezia Giulia. Regione autonoma Friuli Venezia Giulia, Azienda parchi e foreste regionali
Pressey RL, Cabeza M, Watts ME, Cowling RM, Wilson KA (2007) Conservation planning in a changing world. Trends Ecol Evol 22:583–592. https://doi.org/10.1016/j.tree.2007.10.001
Pysek P (1997) Compositae as invaders: better than others? Preslia 69:9–22
Pyšek P (1998) Is there a taxonomic pattern to plant invasions? Oikos 82:282–294. https://doi.org/10.2307/3546968
Pyšek P, Richardson DM (2007) Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W (ed) Biological invasions. Springer, Berlin, Heidelberg, pp 97–125
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raizada P, Raghubanshi AS, Singh JS (2008) Impact of invasive alien plant species on soil processes: a review. Proc Natl Acad Sci India Sect B-Biol Sci 78:288–298
Rejmanek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1655–1661. https://doi.org/10.2307/2265768
Renne IJ, Sinn BT, Shook GW, Sedlacko DM, Dull JR, Villarreal D, Hierro JL (2014) Eavesdropping in plants: delayed germination via biochemical recognition. J Ecol 102:86–94. https://doi.org/10.1111/1365-2745.12189
Rice KJ, Dyer AR (2001) Seed aging, delayed germination and reduced competitive ability in Bromus tectorum. Plant Ecol 155:237–243
Ritz C, Pipper CB, Streibig JC (2013) Analysis of germination data from agricultural experiments. Eur J Agron 45:1–6. https://doi.org/10.1016/j.eja.2012.10.003
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose–response analysis using R. PLoS ONE 10:e0146021. https://doi.org/10.1371/journal.pone.0146021
Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Syst 18:209–235
Roberts EH (1988) Temperature and seed germination. In: Symposia of the Society for Experimental Biology, pp 109–132
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60. https://doi.org/10.1038/nature01333
Ross MA, Harper JL (1972) Occupation of biological space during seedling establishment. J Ecol 60:77–88
Schlau BM, Huxman TE, Mooney KA, Pratt JD (2023) Three-way species interactions reverse the positive pairwise effects of two natives on an exotic invader. Plant Ecol 224:1–11
Stevens PF (2001) Onwards. Angiosperm Phylogeny Website. Version 14, July 2017 [and more or less continuously updated since]. Will do. http://www.mobot.org/MOBOT/research/APweb/. http://www.mobot.org/MOBOT/research/APweb/. Accessed 7 Oct 2022
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:357–374. https://doi.org/10.1111/j.1461-0248.2005.00874.x
Thuiller W, Richardson DM, Midgley GF (2007) Will climate change promote alien plant invasions? In: Nentwig W (ed) Biological invasions. Springer, Berlin Heidelberg, pp 197–211
Tielbörger K, Prasse R (2009) Do seeds sense each other? Testing for density-dependent germination in desert perennial plants. Oikos 118:792–800. https://doi.org/10.1111/j.1600-0706.2008.17175.x
Trotta G, Boscutti F, Jamoneau A, Decocq G, Chiarucci A (2023) There is room for everyone: invasion credit cannot be inferred from the species–area relationship in fragmented forests. Appl Veg Sci 26:e12745. https://doi.org/10.1111/avsc.12745
Turner KG, Hufbauer RA, Rieseberg LH (2014) Rapid evolution of an invasive weed. New Phytol 202:309–321. https://doi.org/10.1111/nph.12634
Vaughn KJ, Young TP (2015) Short-term priority over exotic annuals increases the initial density and longer-term cover of native perennial grasses. Ecol Appl 25:791–799. https://doi.org/10.1890/14-0922.1
Verdú M, Traveset A (2005) Early emergence enhances plant fitness: a phylogenetically controlled meta-analysis. Ecology 86:1385–1394
Vidotto F, Tesio F, Ferrero A (2013) Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop Prot 54:161–167. https://doi.org/10.1016/j.cropro.2013.08.009
Vitti S, Pellegrini E, Casolo V, Trotta G, Boscutti F (2020) Contrasting responses of native and alien plant species to soil properties shed new light on the invasion of dune systems. J Plant Ecol 13:667–675. https://doi.org/10.1093/jpe/rtaa052
Wainwright CE, Cleland EE (2013) Exotic species display greater germination plasticity and higher germination rates than native species across multiple cues. Biol Invasions 15:2253–2264
Wainwright CE, Wolkovich EM, Cleland EE (2012) Seasonal priority effects: implications for invasion and restoration in a semi-arid system. J Appl Ecol 49:234–241. https://doi.org/10.1111/j.1365-2664.2011.02088.x
Wilson JB, Peet RK, Dengler J, Pärtel M (2012) Plant species richness: the world records. J Veg Sci 23:796–802. https://doi.org/10.1111/j.1654-1103.2012.01400.x
Yannelli FA, Koch C, Jeschke JM, Kollmann J (2017) Limiting similarity and Darwin’s naturalization hypothesis: understanding the drivers of biotic resistance against invasive plant species. Oecologia 183:775–784. https://doi.org/10.1007/s00442-016-3798-8
Yu H, Liu J, He W-M, Miao S-L, Dong M (2011) Cuscuta australis restrains three exotic invasive plants and benefits native species. Biol Invasions 13:747–756
Zając M, Zając A, Tokarska-Guzik B (2009) Extinct and endangered archaeophytes and the dynamics of their diversity in Poland. Biodivers Res Conserv 13:17–24
Zhang S, Liu J, Bao X, Niu K (2011) Seed-to-seed potential allelopathic effects between Ligularia virgaurea and native grass species of Tibetan alpine grasslands. Ecol Res 26:47–52. https://doi.org/10.1007/s11284-010-0751-x
Zhao H, Peng S, Wu J, Xiao H, Chen B (2008) Allelopathic potential of native plants on invasive plant Mikania micrantha HBK in South China. Allelopath J 22:189–196
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
FB, EP, GT and MV conceived the ideas and designed methodology; EP, GT and MV has collected all the data; GT and FB analysed the data; GT, FB and EP led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Jaime Moyano.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trotta, G., Vuerich, M., Petrussa, E. et al. Germination performance of alien and native species could shape community assembly of temperate grasslands under different temperature scenarios. Plant Ecol 224, 1097–1111 (2023). https://doi.org/10.1007/s11258-023-01365-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-023-01365-7