Abstract
Background
Increased permeability of the intestinal wall and intestinal dysbiosis may contribute to chronic systemic inflammation, one of the causes of accelerated atherosclerosis and cardiovascular morbidity and mortality burden in patients with chronic kidney disease. The aim of this study was to evaluate the association between markers of intestinal permeability and inflammation in haemodialysis (HD) patients.
Methods
Plasma concentration of zonulin, haptoglobin, TNFα, IL6, d-lactates and bacterial lipopolysaccharides (LPS) was assessed in blood samples obtained after overnight fast before midweek morning HD session in 150 stable, prevalent HD patients. Daily intake of energy and macronutrients was assessed on the basis of a food frequency questionnaire.
Results
Serum hsCRP level was increased in over 70% of patients. Plasma levels of zonulin [11.6 (10.9–12.3) vs 6.8 (5.8–7.8) ng/mL], IL6 [6.2 (1.0–10.3) vs 1.3 (1.0–2.0) pg/mL] and TNFα [5.9 (2.9–11.8) vs 1.6 (1.3–1.8) pg/mL], but not LPS and d-lactates were significantly higher in HD than in healthy controls. d-lactates and LPS levels were weakly associated with IL6 (R = 0.175; p = 0.03, and R = 0.241; p = 0.003). There was a borderline correlation between plasma zonulin and serum hsCRP (R = 0.159; p = 0.07), but not with IL6, LPS and d-lactates. In multiple regression, both serum CRP and plasma IL6 variability were explained by LPS (β = 0.143; p = 0.08 and β = 0.171; p = 0.04, respectively), only.
Conclusion
The weak association between plasma d-lactate, LPS and IL6 levels indicates that intestinal flora overgrowth or increased intestinal permeability contributes very slightly to the chronic inflammation development in HD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The accelerated development of atherosclerosis is one of the main causes of precocious cardiovascular morbidity and mortality in dialysis patients with chronic kidney disease (CKD) [1]. It can be explained by the co-occurrence of classic cardiovascular risk factors (age, sex, hypertension, lipid disorders, diabetes or obesity) and the so-called uraemia-specific risk factors, which include CKD-MBD (chronic kidney disease—mineral and bone disorders), oxidative stress, accumulation of advanced glycation end products (AGEs), uraemic retention solutes (e.g. guanidine, asymmetric dimethylarginine (ADMA), p-cresol, indoxyl sulphate), as well as low-grade chronic systemic inflammation (microinflammation) [2–5].
The pathogenesis of chronic inflammation in haemodialysis (HD) patients is complex and not entirely explained. It is closely related to atherosclerosis by a bidirectional association. Inflammation accelerates the progress of atherosclerosis, and during atherogenesis development, an activation of monocytes and macrophages that secrete pro-inflammatory cytokines (e.g. TNFα) and chemokines occurs. In addition, visceral adipose tissue (the sources of pro-inflammatory cytokines) in patients with abdominal obesity, contact with blood by the dialyser membrane and probably increased intestinal permeability are contributing to increased inflammatory state [6].
During the last few years, the importance of intestinal microflora contributing to the development of autoimmune diseases, hormonal metabolism and systemic inflammation was raised [7, 8]. Impaired structure and function of the intestinal barrier related to dysbiosis may increase the penetration of bacteria-derived toxins (including lipopolysaccharides), or even viable bacteria into the blood. This mechanism can induce systemic inflammation [8, 9]. Accumulation of uraemic toxins and metabolic disorders in CKD patients may influence the composition of intestinal flora and promote gut permeability. That may lead to depletion of immune system mechanisms and finally to the state of immunodeficiency and chronic systemic inflammation; however, the data supporting this hypothesis are limited [6]. Wang et al. [10] found the evidence for intestinal flora translocation (bacterial ribosomal DNA in blood) in six CKD stage 5 patients. The presence of bacterial DNA was also associated with significantly higher levels of highly sensitive CRP, IL6 and d-lactates [11].
Recently zonulin, a new marker potentially useful for the evaluation of the permeability of intestinal wall, was described [12]. Zonulin is a protein (molecular weight of 47 kDa), HP2 gene product (which simultaneously encodes haptoglobin), which interacts with intercellular connections (Zona occludens) [13]. Increased concentration of zonulin was observed in patients with sepsis [14], obesity [7], type 2 diabetes [15] and autoimmune diseases [16]. It has not been established whether synthesis of zonulin is altered in CKD patients.
Additionally, measurement of d-lactate (a product of anaerobic metabolism from intestinal bacteria) concentration in the circulation may reflect colonic absorption of bacterial metabolism products. d-lactate and bacterial endotoxins are considered primarily as markers of colon absorption, partially reflecting the permeability of the intestinal wall [17, 18].
The aim of this study was to evaluate the association between markers of intestinal permeability and systemic inflammation in haemodialysis patients.
Subjects and methods
One hundred and fifty (N = 150) stable, prevalent HD patients (92 men and 58 women) were enrolled into the study. Only patients on morning dialysis sessions were included due to requirement of morning blood samples collection (after overnight fast). Patients with previous history of gastrointestinal diseases, receiving immunosuppressive medication, during hospitalization and on HD therapy for less than 6 months were excluded (Fig. 1). The study protocol was accepted by the Bioethical Committee of Medical University of Silesia in Katowice (KNW-2-015/N/3/K), and each patient gave informed consent for participation in the study. The study protocol neither included training with a nutritionist nor interfered with previous nutrition recommendations.
All HD patients were dialysed 3 times per week for 3.5–5 h (11.7 ± 0.9 h weekly) using low-flux dialysers with polyethersulphone (PES) or polysulphone (PSU) membranes (area range 1.5–2.0 m2), with blood flow rates from 200 to 250 ml/min and dialysate flow rate 500 ml/min. Patient characteristics including CKD causes, duration of HD therapy and Kt/V are given in Table 1.
Thirty subjects (9 women and 21 men) without concomitant diseases: 9 obese and 21 normal weight, served as a control group. The exclusion criteria included acute or chronic diseases, any drug use, including oral contraceptive agents, body mass changes exceeding more than 3 kg during preceding 6 months, cigarette smoking, drinking more than three drinks per week, endocrine disorders: hyper- and hypothyroidism, Cushing’s syndrome, polycystic ovary syndrome.
The study protocol assumed a single withdrawal of an additional blood sample, while performing routine tests (blood count, serum urea, calcium, phosphate, sodium, potassium), before the midweek HD session after overnight fasting. Only patients on morning haemodialysis sessions were recruited. Also in the control group, blood samples were obtained in the morning after overnight fast.
Measurements
Plasma concentrations of zonulin (Immundiagnostik AG, Bensheim, Germany) haptoglobin (AssayPro, Saint Charles, Mo, U.S.), TNFα and IL6 (R&D Systems, Minnesota, MN, U.S.), d-lactates (Uscn Life Sciences Inc., Wuhan, PRC) and bacterial lipopolysaccharides (Uscn Life Sciences Inc., Wuhan, PRC) were assessed by ELISA using commercially available kits. The intra-assay and inter-assay coefficients of variability were as follows: <5 and <8.5% (zonulin), <4.9 and <7.5% (haptoglobin), <7.3 and <4.3% (TNFα), <7.2 and <7.8% (IL6), <8 and <10% (d-lactates), <10 and <12% (lipopolysaccharides).
Serum hsCRP was assessed by immunoturbidimetric method (DRG Instruments GmbH for Hybrid XL, Marburg, Germany) with inter-assay precision <4.8%.
Statistical analysis
Statistical analysis was performed with STATISTICA 10.0 PL StatSoft, Inc. software (www.statsoft.com). The normality of quantitative variables distribution was checked by Shapiro–Wilk test. Results are given as mean values with standard deviation or 95% confidence intervals (95% CI) or medians with interquartile range (variables with skewed distribution). Study population was divided in tertiles based on plasma concentration of zonulin, lipopolysaccharides and d-lactates. For comparison of groups (HD patients and controls, and subgroups), we used the Chi-square test (qualitative variables), and ANOVA or Kruskal–Wallis one-way analysis of variance by ranks followed by Mann–Whitney U test (quantitative variables). Correlation coefficient was calculated according to Spearman.
Value of p < 0.05 was considered statistically significant in all analyses.
Results
Study group characteristics
One hundred and fifty haemodialysis patients were included in the study (more than two-thirds of them were men). The mean duration of dialysis time was 4 years. Average BMI was 26 kg/m2, and 18 (12%) patients met the criteria for obesity. Causes of CKD were typical; the most common cause was diabetes, then glomerulonephritis and finally, hypertensive nephropathy (Table 1). The majority of patients were suffering from arterial hypertension, more than half of them from coronary disease, and one-third from diabetes. Dozens of patients having prior stroke and a similar group had a history of past kidney transplantation. Half of the patients took anti-platelet agents and over one-third statins. Oral phosphorous binders were prescribed for the majority of the patients (n = 129) (Table 1).
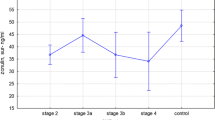
The average value of haemoglobin (10.8 g/dL) suggested effective treatment for patients with anaemia. The mean serum concentration of total and LDL cholesterol was within the normal range, while the average serum HDL concentration was low. Triglyceride concentration exceeded the upper limit; typical for CKD stage 5 calcium–phosphate disturbances with secondary hyperparathyroidism were observed. Inflammatory markers (serum hsCRP and plasma TNFα) were slightly elevated (Table 2). An increased hsCRP level (over 3 mg/dL) was found in over 70% of patients. The levels of zonulin (Fig. 2), hsCRP, IL6 and TNFα were significantly increased in relation to values in control subjects (Table 2). The levels of haptoglobin, LPS and d-lactates were similar in HD and controls.
Zonulin level variability was not explained by residual diuresis, Kt/V and urea reduction ratio.
Subgroups analysis
The zonulin tertiles subgroups of HD patients differ in respect of hsCRP and TNFα, but had similar levels of d-lactates, LPS and haptoglobin. Similar analysis for LPS tertiles showed increasing IL6 levels in the subsequent tertiles (Fig. 3). In addition, d-lactates tertiles differed in respect of IL6 and haptoglobin, but not LPS and zonulin levels (Table 3).
Correlations between LPS, d-lactates and zonulin, and inflammatory markers
There was a stronger correlation between plasma concentrations of LPS and IL6 (R = 0.241; p = 0.003) than with serum hsCRP level (R = 0.153; p = 0.06) (Fig. 4). No association with TNFα was found (R = 0.101; p = 0.22). The levels of d-lactates were weakly associated with IL6 (R = 0.175; p = 0.03), but neither with hsCRP (R = −0.022; p = 0.78) nor with TNFα (R = −0.139; p = 0.09).
There was a borderline correlation between plasma zonulin and serum hsCRP (R = 0.159; p = 0.07). No correlation with plasma IL6 (R = 0.118; p = 0.15), TNFα (R = 0.114; p = 0.17), LPS (R = 0.019; p = 0.82) and d-lactates (R = −0.083; p = 0.31) was found.
The plasma levels of haptoglobin were weakly related to those of zonulin (R = 0.138; p = 0.09) and d-lactates (R = 0.186; p = 0.02).
Multiple regression models
In multiple regression models including plasma concentrations of zonulin, LPS and d-lactates, both serum CRP and plasma IL6, but not plasma TNFα variability was explained only by LPS (β = 0.143; p = 0.08 and β = 0.171; p = 0.04, respectively).
Discussion
The levels of zonulin, hsCRP, IL6 and TNFα were significantly higher in HD patients, while the levels of haptoglobin, LPS and d-lactates were similar in both study groups. Additionally, a weak association between plasma levels of IL6 and d-lactate as well as LPS were observed, while in multiple regression models, serum hsCRP and plasma IL6 variability were explained by LPS, only.
Moderate increase of circulating inflammatory markers (CRP, IL6 and TNFα) is typical for CKD patients [4, 5]. In addition, the relationship between systemic inflammation in CKD patients and the severity of endotoxaemia was reported [19]. Moreover, in animals with experimentally induced CKD, a bacterial DNA was detected in blood, spleen, lymph nodes and the liver [20]. The most likely source of bacteria is the gut microflora. In CKD, impaired intestinal epithelium cellular tight junctions, which allow the passage of endotoxins and bacteria into circulation, were observed [21, 22]. In addition, an altered composition of the intestinal microflora of CKD patients was described. This change can be explained by uraemia and additionally by specific diet and medications used in those patients [10]. However, in our study despite higher zonulin levels in the circulation of HD patients, suggesting increased gut permeability, similar concentrations of LPS and d-lactates were found. In addition, similar concentrations of LPS were showed in zonulin tertiles. These data do not confirm significant endotoxaemia in the majority of HD patients without gastrointestinal diseases and raise the uncertainty concerning zonulin as the marker of gut permeability in HD. It should be noted that until now, plasma zonulin (prehaptoglobin-2) concentration has not been evaluated in HD patients. Our study shows elevated plasma levels of zonulin in these patients even after comparison with obese subjects [8.2 (7.1–8.4) ng/mL] [7]. The cause of increased zonulin level in HD is unclear, also taking into account the lack of elevation of plasma haptoglobin (encoded by the same gene as zonulin). Perhaps increased plasma zonulin levels in HD result from decreased degradation/elimination by the kidneys.
One of the examined parameters was plasma level of d-lactic acid, a surrogate marker of colon bacteria overgrowth. d-lactic acid exists as two stereoisomers: right-handed (d) and left-handed (l). Both isomers, as a result of the action of a specific lactate dehydrogenase (l and d) (via pyruvate conversion), d- or l-lactic acid molecule is produced. In human cells, activity of d-lactate dehydrogenase is not observed, and naturally, only the l-form of lactic acid occurs. However, d-lactic acid can be present in the blood stream and reflect exogenous origin or be the result of methylglyoxal metabolism. Methylglyoxal may be derived from carbohydrates, fatty acid or amino acids transformation. It is a toxic compound and therefore, via glyoxalase 1 and glyoxalase 2, is converted to d-lactate. Furthermore, d-lactates can be derived from enteric bacterial fermentation predominantly of Lactobacillus and Bifidobacteria [23].
Increased plasma concentration of d-lactates may occur in the case of sugar overabundance in the diet, short bowel syndrome, as well as microbial proliferation and excessive fermentation [23]. It is not clear whether increased intestinal permeability can be accompanied by progression of kidney disease or can be due to influence of uraemic toxins on the intestinal wall. Some studies indicate the role of urea in this process, but it does not seem to be a key element in the development of chronic microinflammation. Considering the role of intestinal microflora in the development of autoimmune diseases and systemic inflammation shown in clinical trials [7], we design this study to explain whether chronic inflammation in HD patients corresponds to increased intestinal permeability and uraemia-related dysbiosis. However, in our study, plasma levels of d-lactates were similar in HD and controls and were weakly associated with plasma IL6 concentration. Thus, it seems that microbial proliferation and excessive fermentation in the colon are only mild factors participating in the systemic microinflammation in HD patients.
A weak correlation between levels of IL6 and d-lactates and LPS, and the greatest severity of inflammation were found in the highest tertile of zonulin, LPS and d-lactates concentrations, while in multiple regression models, serum hsCRP and plasma IL6 variability were explained by LPS, only. This supports the hypothesis that bacteria overgrowth in the gut or the increased permeability of intestinal wall only in a small part participates to the inflammation development in HD patients. In addition, the weakness of these relationships confirms that the other well-known mechanisms of systemic inflammation in HD [2] have a predominant role.
The study has some limitations, including the lack of the intestinal microflora analysis and the absence of microflora bacterial (ribosomal) DNA identification in plasma samples, and the lack of performance of intestinal permeability tests. Furthermore, the cross-sectional study design precludes the analysis of cause-and-effect relations.
Conclusion
The weak association between plasma d-lactate, LPS and IL6 levels indicates that intestinal flora overgrowth or increased intestinal permeability contributes very slightly to the chronic inflammation development in HD patients.
References
Lindner A, Charra B, Sherrard DJ, Scribner BH (1974) Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med 290:697–701
Wanner C, Zimmermann J, Schwedler S, Metzger T (2002) Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl 80:99–102
Johnson RJ, Feehally J, Floege J (2014) Comprehensive clinical nephrology, 5th edn. Elsevier Health Sciences, Saunders, Amsterdam, pp 916–1029
Pecoits-Filho R, Bárány P, Lindholm B, Heimbürger O, Stenvinkel P (2002) Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17:1684–1688
Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS; CRIC Study Investigators (2012) Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 7:1938–1946
Anders HJ, Andersen K, Stecher B (2013) The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 83:1010–1016
Żak-Gołąb A, Kocełak P, Aptekorz M, Zientara M, Juszczyk L, Martirosian G, Chudek J, Olszanecka-Glinianowicz M (2013) Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Int J Endocrinol 2013:674106. doi:10.1155/2013/674106
Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD (2011) Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 7:639–646
Wirostko E, Johnson L, Wirostko B (1990) Ulcerative colitis associated chronic uveitis. Parasitization of intraocular leucocytes by mollicute-like organisms. J Submicrosc Cytol Pathol 22:231–239
Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S (2012) Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton) 17:733–738
Shi K, Wang F, Jiang H, Liu H, Wei M, Wang Z, Xie L (2014) Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci 59:2109–2117
Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE (2000) Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 355:1518–1519
Smecuol E, Sugai E, Niveloni S, Vázquez H, Pedreira S, Mazure R, Moreno ML, Label M, Mauriño E, Fasano A, Meddings J, Bai JC (2005) Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol 3:335–341
Klaus DA, Motal MC, Burger-Klepp U, Marschalek C, Schmidt EM, Lebherz-Eichinger D, Krenn CG, Roth GA (2013) Increased plasma zonulin in patients with sepsis. Biochem Med (Zagreb) 23:107–111
Zhang D, Zhang L, Zheng Y, Yue F, Russell RD, Zeng Y (2014) Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res Clin Pract 106:312–318
Pabijasz D, Grzybowska-Chlebowczyk U, Woś H (2013) Evaluation of intestinal permeability on the basis of zonulin levels, in children with inflammatory bowel disease. Post Nauk Med 5:346–350
Ruan P, Gong ZJ, Zhang QR (2004) Changes of plasma d(-)-lactate, diamine oxidase and endotoxin in patients with liver cirrhosis. Hepatobiliary Pancreat Dis Int 3:58–61
Smith SM, Eng RH, Campos JM, Chmel H (1989) d-lactic acid measurements in the diagnosis of bacterial infections. J Clin Microbiol 27:385–388
Kotanko P, Carter M, Levin NW (2006) Intestinal bacterial microflora—a potential source of chronic inflammation in patients with chronic kidney disease. Nephrol Dial Transplant 21:2057–2060
Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C (1999) Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 55:648–658
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83:308–315
Vaziri ND, Dure-Smith B, Miller R, Mirahmadi MK (1985) Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am J Gastroenterol 80:608–611
Ewaschuk JB, Naylor JM, Zello GA (2005) d-lactate in human and ruminant metabolism. J Nutr 135:1619–1625
Funding
The study was covered by Grants from Medical University of Silesia (KNW-1-062/N/4/0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest, financial or otherwise.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ficek, J., Wyskida, K., Ficek, R. et al. Relationship between plasma levels of zonulin, bacterial lipopolysaccharides, d-lactate and markers of inflammation in haemodialysis patients. Int Urol Nephrol 49, 717–725 (2017). https://doi.org/10.1007/s11255-016-1495-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1495-5