Abstract
Background
Mycophenolate mofetil (MMF) has shown to be a reliable choice in the treatment of glomerulonephritis. We retrospectively reviewed the clinical course and response to MMF therapy in 49 patients with primary glomerulopathy (37 patients) and lupus nephritis [class III (five patients) and IV (seven patients)].
Methods
Patients were treated with MMF for more than 6 months as a primary (18 patients) or an adjunctive treatment (31 patients). Patients were also on methylprednisolone (2–20 mg/day) and angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
Results
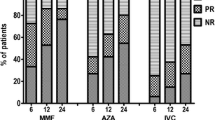
The mean age of the patient cohort was 33.69 ± 12.4 years (range 19–59 years). Twenty-four-hour urinary protein excretion was reduced from 3.50 ± 3.08 g prior to the commencement of MMF drug therapy to 1.21 ± 1.44 and 0.99 ± 1.34 g at the sixth and 12th months of MMF therapy, respectively (P < 0.05 for all). During this same period, significant increases in serum total protein (from 5.92 ± 1.38 to 6.59 ± 0.79 and 6.81 ± 0.77 g/dl) and albumin levels (from 3.23 ± 1.10 to 3.93 ± 0.67 and 4.21 ± 0.50 g/dl) were detected, whereas total cholesterol and low-density lipoprotein levels were found to be significantly decreased (P < 0.05 for all). Serum creatinine levels did not significantly change. The efficacy of MMF in reducing proteinuria was similar in both first line and an adjunctive therapy. The efficacy of MMF therapy began at the third month of treatment and continued through to the 12th month.
Conclusion
Mycophenolate mofetil therapy was found to be useful in achieving improvements in proteinuria and nephrotic syndrome and stabilizing renal function. It was also a well-tolerated drug by the majority of the patients. Based on our results, we suggest that MMF may be alternative therapy for resistant/relapsing primary glomerulopathies and lupus nephritis.


Similar content being viewed by others
References
Eugui EM, Almquist SJ, Muller CD, Allison AC (1991) Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand J Immunol 33:161–173. doi:10.1111/j.1365-3083.1991.tb03746.x
Allison AC, Eugut EM, Sollinger HW (1993) Mycophenolate mofetil (RS-61443): mechanism of action and effects in transplantation. Transpl Rev 7:129–139. doi:10.1016/S0955-470X(05)80012-6
Allison AC (1994) Preferential suppression of lymphocyte proliferation by mycophenolic acid and predicted long-term effects of mycophenolate mofetil in transplantation. Transpl Proc 26:3205–3210
Allison AC, Kowalski WJ, Muller CJ et al (1993) Mycophenolic acid and brequinar, inhibitors of purine and pyrimidine synthesis, block the glycosylation of adhesion molecules. Transpl Proc 25[Suppl 2]:67–70
Chang C-CJ, Aversa G, Punnonen J et al (1993) Brequinar sodium, mycophenolic acid, and cyclosporin A inhibit different stages of IL-4 or IL 13 d human IgG4 and IgE production in vitro. Ann N Y Acad Sci 696:108–122
Blaheta RA, Leckel K, Wittig B, Zenker D, Oppermann E, Harder S et al (1999) Mycophenolate mofetil impairs transendothelial migration of allogeneic CD4 and CD8 T-cells. Transpl Proc 31:1250–1252. doi:10.1016/S0041-1345(98)01984-8
Sollinger HW (1995) Mycophenolate mofetil for prevention of acute rejection in primary cadaveric renal allograft recipients. US Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 60:225–232. doi:10.1097/00007890-199508000-00003
The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group (1996) A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 61:1029–1037. doi:10.1097/00007890-199604150-00008
Sheashaa HA, Bakr MA, Fouda MA, El-Dahshan KF, Ismail AM, Sobh MA et al (2007) Is it worth using daclizumab induction therapy with mycophenolate mofetil-based immunosuppressive regimens in live related donor kidney transplantation? A long-term follow up. Int Urol Nephrol 39(1):317–319. doi:10.1007/s11255-006-9091-8
Ojo AO, Meier-Kriesche HU, Hanson JA et al (2000) Mycophenolate mofetil reduces late renal allograft loss of acute rejection. Transplantation 69:2405–2409. doi:10.1097/00007890-200006150-00033
Mok CC, Lai KN (2002) Mycophenolate mofetil in lupus glomerulonephritis. Am J Kidney Dis 40:447–457. doi:10.1053/ajkd.2002.34882
Karim MY, Pisoni CN, Ferro L, Tungekar MF, Abbs IC, D’Cruz DP et al (2005) Reduction of proteinuria with mycophenolate mofetil in predominantly membranous lupus nephropathy. Rheumatology (Oxford) 44:1317–1321. doi:10.1093/rheumatology/kei019
Dooley MA, Cosio FG, Nachman PH, Falkenhain ME, Hogan SL, Falk RJ et al (1999) Mycophenolate mofetil therapy in lupus nephritis: clinical observations. J Am Soc Nephrol 10:833–839
Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH et al (2005) Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int 68:802–812. doi:10.1111/j.1523-1755.2005.00460.x
Frutos MA, Lopez V, Alferez MJ, Aranda P (2005) Mycophenolate mofetil in high risk IgA glomerulonephritis. Nefrologia 25:387–392
El-Reshaid K, El-Reshaid W, Madda J (2005) Combination of immunosuppressive agents in treatment of steroid-resistant minimal change disease and primary focal segmental glomerulosclerosis. Ren Fail 27:523–530. doi:10.1080/08860220500198623
Choi MJ, Eustace JA, Gimenez LF, Atta MG, Scheel PJ, Sothinathan R et al (2002) Mycophenolate mofetil treatment for primary glomerular diseases. Kidney Int 61:1098–1114. doi:10.1046/j.1523-1755.2002.00214.x
Segarra A, Amoedo ML, Martinez Garcia JM, Pons S, Praga M, Garcia EI et al (2007) Efficacy and safety of ‘rescue therapy’ with mycophenolate mofetil in resistant primary glomerulonephritis—a multicenter study. Nephrol Dial Transpl 22(5):1351–1360. doi:10.1093/ndt/gfl805
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paydas, S., Kurt, C., Taskapan, H. et al. The effect of mycophenolate mofetil on primary and secondary treatment of primary glomerulonephritis and lupus nephritis. Int Urol Nephrol 41, 145–152 (2009). https://doi.org/10.1007/s11255-008-9454-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-008-9454-4