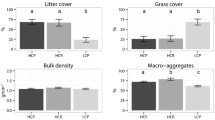
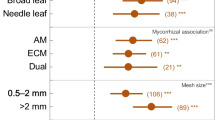
Abstract
Functional diversity and composition of soil bacterial communities affect important soil biogeochemical processes. In natural and semi-natural ecosystems, variations in habitat complexity have been shown to significantly impact both litter and soil bacterial communities. However, this remains largely untested in urban ecosystems, where human management can lead to habitat complexity combinations unobserved in rural ecosystems. We established 10 research plots in low-complexity park, high-complexity park, and high-complexity remnant habitat types (n = 30) in Melbourne, Australia. The use of organic carbon substrates by soil and litter bacteria was measured using EcoPlates to investigate the effects of habitat complexity upon metabolic functional diversity and functional composition of bacterial communities of i) soil and ii) one-year old litter. Direct and indirect effects of habitat complexity, microclimate and decomposition status upon litter microbial functional diversity and composition were also modelled using path analysis. Soil bacterial communities had significantly higher functional diversity compared to litter bacterial communities, but no significant effect of habitat complexity was apparent. The functional composition of soil bacterial communities was not affected by habitat complexity. In contrast, the functional composition of litter bacterial communities in high complexity parks and remnants was significantly different from that in low-complexity parks. The functional composition of litter bacterial communities, but not their diversity, was directly affected by habitat complexity and microclimate as well as their indirect effects upon the decomposition status of litter. Human management of urban habitat complexity can alter the functional composition of litter and soil bacterial communities without affecting their functional diversity. While this can have significant impacts on bacteria-regulated processes and ecosystem services, it also suggests that urban bacterial communities might be able to adjust to further environmental and climatic changes affecting urban ecosystems.




Similar content being viewed by others
References
Allison SD, Lu Y, Weihe C, Goulden ML, Martiny AC, Treseder KK, Martiny JBH (2013) Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 94:714–725
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Aronson MFJ, La Sorte FA, Nilon CH, Katti M, Goddard MA, Lepczyk CA, Warren PS, Williams NSG, Cilliers S, Clarkson B, Dobbs C, Dolan R, Hedblom M, Klotz S, Louwe Kooijmans J, Kühn I, MacGregor-Fors I, McDonnell M, Mörtberg U, Pyšek P, Siebert S, Sushinsky J, Werner P, Winter M (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc Lond B 281:20133330
Balser TC, Firestone MK (2005) Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73:395–415
Barberán A, Ramirez KS, Leff JW, Bradford MA, Wall DH, Fierer N (2014) Why are some microbes more ubiquitous than others? Predicting the habitat breadth of soil bacteria. Ecol Lett 17:794–802
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: linear mixed-effects models using Eigen and S4. R package version 1.1–7.
Bell T, Newman JA, Silverman BW, Turner SL, Lilley AK (2005) The contribution of species richness and composition to bacterial services. Nature 436:1157–1160
Berthrong ST, Schadt CW, Piñeiro G, Jackson RB (2009) Afforestation alters the composition of functional genes in soil and biogeochemical processes in south American grasslands. Appl Environ Microbiol 75:6240–6248
Bray SR, Kitajima K, Mack MC (2012) Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biol Biochem 49:30–37
Brudvig LA, Grman E, Habeck CW, Orrock JL, Ledvina JA (2013) Strong legacy of agricultural land use on soils and understory plant communities in longleaf pine woodlands. For Ecol Manag 310:944–955
Buesing N, Gessner MO (2002) Comparison of detachment procedures for direct counts of bacteria associated with sediment particles, plant litter and epiphytic biofilms. Acquat Microb Ecol 27:29–36
Byrne LB (2007) Habitat structure: a fundamental concept and framework for urban soil ecology. Urban Ecosyst 10:255–274
Byrne LB, Bruns MA, Kim KC (2008) Ecosystem properties of urban land covers at the aboveground-belowground interface. Ecosystems 11:1065–1077
Cantrell SA, Molina M, Jean Lodge D, Rivera-Figueroa FJ, Ortiz-Hernández ML, Marchetti AA, Cyterski MJ, Pérez-Jiménez JR (2014) Effects of a simulated hurricane disturbance on forest floor microbial communities. For Ecol Manag 332:22–31
Chen X-L, Wang D, Chen X, Wang J, Diao J-J, Zhang J-Y, Guan Q-W (2015) Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl Soil Ecol 92:35–44
DeAngelis KM, Chivian D, Fortney JL, Arkin AP, Simmons B, Hazen TC, Silver WL (2013) Changes in microbial dynamics during long-term decomposition in tropical forests. Soil Biol Biochem 66:60–68
Degens BP (1998) Decreases in microbial functional diversity do not result in corresponding changes in decomposition under different moisture conditions. Soil Biol Biochem 30:1989–2000
Fichtner A, von Oheimb G, Härdtle W, Wilken C, Gutknecht JLM (2014) Effects of anthropogenic disturbances on soil microbial communities in oak forests persist for more than 100 years. Soil Biol Biochem 70:79–87
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631
Fierer N, Breitbart M, Nulton J, Salamon P, Lozupone C, Jones R, Robeson M, Edwards RA, Felts B, Rayhawk S, Knight R, Rohwer F, Jackson RB (2007) Metagenomic and small-subunit rRNA analyses reveal the genetic diversity of bacteria, archaea, fungi, and viruses in soil. Appl Environ Microbiol 73:7059–7066
Fioretto A, Papa S, Curcio E, Sorrentino G, Fuggi A (2000) Enzyme dynamics on decomposing leaf litter of Cistus incanus and Myrtus communis in a Mediterranean ecosystem. Soil Biol Biochem 32:1847–1855
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Chang Biol 6:751–765
Giai C, Boerner REJ (2007) Effects of ecological restoration on microbial activity, microbial functional diversity, and soil organic matter in mixed-oak forests of southern Ohio, USA. Appl Soil Ecol 35:281–290
Girvan MS, Bullimore J, Pretty JN, Osborn AM, Ball AS (2003) Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl Environ Microbiol 69:1800–1809
González G, Lodge DJ, Richardson BA, Richardson MJ (2014) A canopy trimming experiment in Puerto Rico: the response of litter decomposition and nutrient release to canopy opening and debris deposition in a subtropical wet forest. For Ecol Manag 332:32–46
Gough C, Elliott HL (2012) Lawn soil carbon storage in abandoned residential properties: an examination of ecosystem structure and function following partial human-natural decoupling. J Environ Manag 98:155–162
Hättenschwiler S, Fromin N, Barantal S (2011) Functional diversity of terrestrial microbial decomposers and their substrates. C R Biol 334:393–402
Hortal S, Bastida F, Armas C, Lozano YM, Moreno JL, García C, Pugnaire FI (2013) Soil microbial community under a nurse-plant species changes in composition, biomass and activity as the nurse grows. Soil Biol Biochem 64:139–146
Kendrick JA, Ribbons RR, Classen AT, Ellison AM (2015) Changes in canopy structure and ant assemblages affect soil ecosystem variables as a foundation species declines. Ecosphere 6:art 77
Kim M, Kim W-S, Tripathi B, Adams J (2014) Distinct bacterial communities dominate tropical and temperate zone leaf litter. Microb Ecol 67:837–848
Koehler HH (2000) Natural regeneration and succession - results from a 13 years study with reference to mesofauna and vegetation, and implications for management. Landsc Urban Plan 51:123–130
Korb JE, Bombaci S, Siegel R (2014) The effect of sudden aspen decline on understory microclimate and vegetation in southwestern Colorado. Can J For Res 44:914–921
Leflaive J, Céréghino R, Danger M, Lacroix G, Ten-Hage L (2005) Assessment of self-organizing maps to analyze sole-carbon source utilization profiles. J Microbiol Methods 62:89–102
Leflaive J, Danger M, Lacroix G, Lyautey E, Oumarou C, Ten-Hage L (2008) Nutrient effects on the genetic and functional diversity of aquatic bacterial communities. FEMS Microbiol Ecol 66:379–390
Li Q, Lee Allen H, Wollum Ii AG (2004) Microbial biomass and bacterial functional diversity in forest soils: effects of organic matter removal, compaction, and vegetation control. Soil Biol Biochem 36:571–579
Lin WR, Chen WC, Wang PH (2011) Effects of forest thinning on diversity and function of macrofungi and soil microbes. Sydowia 63:67–77
Livesley SJ, Ossola A, Threlfall C, Hahs AK, Williams NSG (2016) Soil carbon and carbon/nitrogen ratio change under tree canopy, tall grass and turf grass areas of urban green spaces. J Environ Qual 45:215–223
Marschner P, Umar S, Baumann K (2011) The microbial community composition changes rapidly in the early stages of decomposition of wheat residue. Soil Biol Biochem 43:445–451
Matulich KL, Martiny JBH (2015) Microbial composition alters the response of litter decomposition to environmental change. Ecology 96:154–163
McGuire KL, Treseder KK (2010) Microbial communities and their relevance for ecosystem models: decomposition as a case study. Soil Biol Biochem 42:529–535
Miki T, Yokokawa T, Matsui K (2013) Biodiversity and multifunctionality in a microbial community: a novel theoretical approach to quantify functional redundancy. Proc R Soc Lond [Biol] 281:20132498
Millward AA, Kamal P, Briggs SE (2011) Naturalization as a strategy for improving soil physical characteristics in a forested urban park. Urban Ecosyst 14:261–278
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
Ngao J, Bernhard-Reversat F, Loumeto J-J (2009) Changes in eucalypt litter quality during the first three months of field decomposition in a Congolese plantation. Appl Soil Ecol 42:191–199
Oksanen J, Guillaume Blanchet F, Kindt R, Legendre P, Minchin P, O'Hara R, Simpson G, Solymos P, Stevens M, Wagner H (2014) Vegan: community ecology package. R package version 2.2–0
Ossola A, Hahs AK, Livesley SJ (2015a) Habitat complexity influences fine scale hydrological processes and the incidence of stormwater runoff in managed urban ecosystems. J Environ Manag 159:1–10
Ossola A, Nash MA, Christie F, Hahs AK, Livesley SJ (2015b) Urban habitat complexity affects species richness but not environmental filtering of morphologically diverse ants. PeerJ 3:e1356
Ossola A, Hahs AK, Nash MA, Livesley SJ (2016) Habitat complexity enhances comminution and decomposition processes in urban ecosystems. Ecosystems 19(5):927–941
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2015). Nlme: linear and nonlinear mixed effects models. R package version 3.1–120
Prescott CE, Grayston SJ (2013) Tree species influence on microbial communities in litter and soil: current knowledge and research needs. For Ecol Manag 309:19–27
Preston-Mafham J, Boddy L, Randerson PF (2002) Analysis of microbial community functional diversity using sole-carbon-source utilisation profiles - a critique. FEMS Microbiol Ecol 42:1–4
R Core Team (2012). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ribeiro C, Madeira M, Araújo MC (2002) Decomposition and nutrient release from leaf litter of Eucalyptus globulus grown under different water and nutrient regimes. For Ecol Manag 171:31–41
Rinkes Z, DeForest J, Grandy AS, Moorhead D, Weintraub M (2014) Interactions between leaf litter quality, particle size, and microbial community during the earliest stage of decay. Biogeochemistry 117:153–168
Sanchez G (2013) PLS path modeling with R. Trowchez Editions, Berkeley
Sanchez G, Trinchera L, Russolillo G (2015) Tools for partial least squares path modeling (PLS-PM). R Package, version 0.4.7
Savva Y, Szlavecz K, Pouyat RV, Groffman PM, Heisler G (2010) Effects of land use and vegetation cover on soil temperature in an urban ecosystem. Soil Sci Soc Am J 74:469–480
Sayer EJ (2006) Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol Rev 81:1–31
Setälä H, McLean MA (2004) Decomposition rate of organic substrates in relation to the species diversity of soil saprophytic fungi. Oecologia 139:98–107
Smith AP, Marín-Spiotta E, Balser T (2015) Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: a multiyear study. Glob Chang Biol 21:3532–3547
Šnajdr J, Cajthaml T, Valášková V, Merhautová V, Petránková M, Spetz P, Leppänen K, Baldrian P (2011) Transformation of Quercus petraea litter: successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol Ecol 75:291–303
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology 90:441–451
Threlfall GC, Ossola A, Hahs AK, Williams NSG, Wilson L, Livesley SJ (2016) Variation in vegetation structure and composition across urban green space types. Front Ecol Evol 4:66
Voriskova J, Baldrian P (2013) Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J 7:477–486
Weber KP, Legge RL (2010). Community-level physiological profiling. In: Cummings SP Ed., Bioremediation, Methods in Molecular Biology. Humana Press. pp 263–281
Weber KP, Grove JA, Gehder M, Anderson WA, Legge RL (2007) Data transformations in the analysis of community-level substrate utilization data from microplates. J Microbiol Methods 69:461–469
Wohl DL, Arora S, Gladstone JR (2004) Functional redundancy supports biodiversity and ecosystem function in a closed and constant environment. Ecology 85:1534–1540
Wu J, Liu Z, Wang X, Sun Y, Zhou L, Lin Y, Fu S (2011) Effects of understory removal and tree girdling on soil microbial community composition and litter decomposition in two eucalyptus plantations in South China. Funct Ecol 25:921–931
Yao H, Bowman D, Shi W (2006) Soil microbial community structure and diversity in a turfgrass chronosequence: land-use change versus turfgrass management. Appl Soil Ecol 34:209–218
Zak JC, Willig MR, Moorhead DL, Wildman HG (1994) Functional diversity of microbial communities: a quantitative approach. Soil Biol Biochem 26:1101–1108
Zhao D, Li F, Yang Q, Wang R, Song Y, Tao Y (2013) The influence of different types of urban land use on soil microbial biomass and functional diversity in Beijing, China. Soil Use Manag 29:230–239
Acknowledgments
This project was funded by the Australian Research Council (ARC LP 110100686), the Australian Research Centre for Urban Ecology (ARCUE) at the Royal Botanic Gardens Victoria, and the Australian Golf Course Superintendents’ Association (AGCSA). Authors declare no conflict of interest. AO, CA, AKH and SJL conceived of the study design. AO performed research. AO and CA analysed data. AO wrote the paper with scientific and editorial review by CA, AKH and SJL. AO was supported by MIFRS and MIRS scholarships, the David Lachlan Hay Memorial Fund and an US National Academy of Sciences, Engineering and Medicine NRC Associateship. Belinda Lees, Carolyn Selway and Vince Kalangi provided valuable laboratory assistance. Authors are also grateful to the AGSCA Members and the Municipalities of Kingston, Frankston and Greater Dandenong for their collaboration. The editor and reviewers of an earlier version of this manuscript are also acknowledged for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ossola, A., Aponte, C., Hahs, A.K. et al. Contrasting effects of urban habitat complexity on metabolic functional diversity and composition of litter and soil bacterial communities. Urban Ecosyst 20, 595–607 (2017). https://doi.org/10.1007/s11252-016-0617-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-016-0617-2