Abstract
The tribocorrosion behaviour of Ti-6Al-4 V exposed to phosphate buffered saline was investigated under a range of normal forces in both cathodic and anodic regions to provide a basis for properly deriving the tribological constants of this alloy. To achieve this, a new customised tribotester was designed and manufactured to rub the Ti-6Al-4 V disks against zirconia balls. The tests were conducted at a sliding frequency of 1 Hz and a sliding distance of 4.8 mm under various normal forces and potentials as 17.5, 10.8, 6, 3.5 N, and − 1.2, − 0.6, 0, 0.4, 0.8 V/VAg/AgCl, respectively. The damaged surfaces were characterised by scanning electron microscopy and energy-dispersive X-ray spectroscopy, profilometer, and micro-hardness tester. The post analyses confirmed the appearance of some minor cracks together with third-body wear particles. No significant changes in the hardness were detected after the tribocorrosion tests. The results of profilometry and electrochemical current indicated that in the anodic region the chemical losses accounted for a significant proportion (up to 36%) of the total loss. The proportional chemical loss increased with the potential; however, neither direct nor reverse relationship was found with the normal force. Overall, in the anodic domain, the material loss increased with the potential level due to the formation of oxide layer which may induce more shear cutting. In the cathodic domain, hydrogen embrittlement changed the properties of the interface and thus, the amount of material loss. Both the mechanical and chemical wear were described by an existing tribocorrosion theory; thereby, the theory was equipped with its tribocorrosive constants for future analyses on the tribocorrosion of this alloy extensively used in various applications including biomedical implants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium-based alloys such as Ti-6Al-4 V are extensively used in various applications including biomedical implants because of their unique mechanical and chemical characteristics (e.g. high specific strength, exceptional corrosion resistance, and noble biocompatibility) [1,2,3,4]. The high corrosion resistance of titanium alloys against a corrosive environment is basically attained by a thin passive oxide layer (usually TiO2) formed spontaneously onto the substrate. Although this layer shields the -substrate against the chemical attacks from the corrosive environment, it possesses inferior mechanical properties which intensifies the total surface damage in mechanically assisted corrosion situations including tribocorrosion [4]. One cycle of tribocorrosion begins with the mechanical abrasion of the oxide layer (depassivation) and finishes with the reformation of the oxide layer (repassivation). Through the cyclic actions of these two mechanisms and their interrelations, the material surface in contact is damaged/tribocorroded. The positive/negative synergy between these mechanisms depends on various parameters such as normal force [5,6,7], sliding distance and its frequency [8,9,10,11], electrochemical potential [12, 13], solution type and its pH [4, 14], and also the material couple in contact [15,16,17].
Tribocorrosion of Ti-6Al-4 V (used extensively for manufacturing biomedical implants) has been reported to cause inflammation which may ultimately lead to implant failure [18,19,20,21,22]. A number of studies published in the literature have investigated the role of oxidation in the tribocorrosion of Ti-6Al-4 V. Runa et al. [12] studied the tribocorrosion of this alloy in phosphate buffered saline (PBS) with a key focus on the role of proteins present in the solution under different anodic potentials. The stability of the oxide layer formed under different potentials was identified as an underlying mechanism leading to a change in the tribological behaviour of the interface. At a potential of − 0.1 V/SCE, a decrease in the electrochemical reactions and the total loss was observed by adding albumin to PBS. For a potential of 0.5 V/SCE, the presence of albumin however increased the total loss from the interface. This result disagrees with findings published in Dimah et al. [23] who reported a decrease in the total loss in the presence of albumin, for a potential of 0.5 V/VAg/AgCl. This disagreement may be attributed to the various mechanical and/or electrochemical parameters used in these research studies. By adding H2O2 to PBS + albumin solution, Gopal and Manivasagam [24] observed a shift in the open circuit potential (OCP) towards more positive values and overall, smaller wear tracks on Ti-6Al-4 V were observed.
In regards to the mechanical aspect, Namus et al. [10] noticed a substantial influence of sliding frequency on the material loss of Ti-6Al-4 V submerged into PBS. This has been also observed in other studies where an increase in sliding frequency resulted in greater material loss whether this loss is more originated from the passivation kinetics and/or the mechanical wear [8, 9]. Considering the passivation phenomenon as a logarithmic/square root function of elapsed time [25]; and also, the low loading frequency in many tribological applications, the oxidation process seems to be completed throughout a stroke of the tribocorrosion cycle. Therefore, most of the previous studies have investigated tribocorrosion under low sliding frequencies [26, 27]. In addition to the sliding frequency, normal force at the interface is another important mechanical parameter which can influence the tribocorrosion damage [28, 29]. This parameter affects both the volume loss from the substrate; and also, the oxidation rate. Previous papers report a direct relationship between the normal force (contact pressure) and total material loss in the tribocorrosion of Ti-6Al-4 V and other metal alloys [5, 7, 29, 30]. Yazdi et al. [29] found a reduction in the “specific tribocorrosion rate (STR)” (defined as the rate of material removal divided by corresponding normal force) with an increase in the normal force. This decrease was believed to be because of the higher coverage of the oxide layer and a decrease in adhesion between the mating surfaces. It was also believed that this decrease could be attributed to an enhancement in the hardness of the wear track, and possible sub-surface grain refinements resulted from work hardening and subsequent plastic deformations onto the surface during the tribocorrosion cycles. However, the enhancement in the wear resistance of Ti-6Al-4 V was not observed elsewhere [31] after the shot-peening process by which work hardening and plastic deformations are induced onto the surface. This necessitates a need for further research to be conducted around the effect of normal force (and subsequent changes in the material properties) on the tribocorrosion of Ti-6Al-4 V alloy. Yazdi et al. [29] also observed a shift in OCP towards negative potentials by increasing the level of normal force. This finding has been also reported by Mardare et al. [6]. The shift might be taken as another source of change in the material loss since it creates an electrochemical situation through which more negative potentials induce higher passivation rates to protect the metal surface.
The relationship between the normal force and material loss can be affected by various parameters such as the level of applied potential. Therefore, the influence of normal force under a reasonable range of imposed potentials on the tribological behaviour of Ti-6Al-4 V needs to be investigated to obtain a better insight into the role of both the mechanical and chemical wear mechanisms in this alloy. The previous studies on Ti-6Al-4 V (coupled with a ceramic counterpart) within PBS were limited to a number of tests in which only either normal force or potential was investigated as a key variable. To the best of the authors’ knowledge, there is no study in the literature investigating the effect of normal force within both the cathodic and anodic domains for this material combination in PBS. Consequently, one cannot theoretically extract the tribological constants which can then be embedded into the tribocorrosion models. These models together with their constants can then be used for predicting the tribocorrosion behaviour and material loss in a nonlinear industrial problem with simulation procedures (whether this nonlinearity is inherited by the geometry, material, and applied loads) [32]. Considering both the extensive use of Ti-6Al-4 V in various industrial sectors and also the costs and risks associated with its tribocorrosion failure, a set of integrated data (under various potentials and normal forces) is essentially required to determine the necessary constants for tribocorrosion models which can be beneficially used in various applications. The present study aims to evaluate the tribocorrosion behaviour of Ti-6Al-4 V under various potentials and normal forces simultaneously. Determining the material volume loss as a measure for surface damage, the roles played by the mechanical and chemical wear mechanisms are aimed to be distinguished. Furthermore, the paper aims to determine the tribological constants for the alloy to more reliably describe the tribological behaviour of Ti-6Al-4 V using an existing model which can then be used for future analyses.
2 Experimental Procedures
2.1 Development of a Tribotester
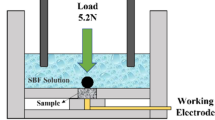
A customised ball-on-disk tribotester was designed and constructed to conduct the tribocorrosion tests for this study (Fig. 1). The designed tribotester is comprised a high-load electromagnetic actuator (LinMot; Model: P10-70 × 400, USA) to generate and control a reciprocating motion at the contacting ball-on-disk interface, a compression-tension fatigue resistant load-cell (Transducer Techniques; Model: SWP-5000, USA) for measuring and recording shear loads induced at the interface, an electrochemically isolated chamber for housing a corrosive solution, a ball gripper mated with a plastic coupler and a plastic cap to isolate the metallic disk from the rest of the metallic parts, a counterbalance mechanism for adjusting the normal force, and a three-axis load-cell (Anhui Zhimin Electrical Technology; Model: ZM3DW, China) to measure and record the normal and shear forces acting at the contacting interface. The linear actuator is capable of generating sliding strokes of up to 50 mm with a desired waveform profile (sinusoidal, triangular, square, etc.). This actuator can produce high-frequency oscillations (maximum velocity and acceleration up to of 4.7 m/s and 975 m/s2, respectively) with a position resolution of 0.1 µm. The sliding stroke is transmitted from the actuator’s stator directly to the chamber which is mounted and rigidly connected to a sliding system. The actual position can be controlled with the actuator's controlling software (LinMot Talk, Version 6.9). The disks (of any material) can be inserted into the chamber and the disk's counterpart (i.e. the ball) is then fastened through the ball gripper connected to the bottom face of the three-axis load-cell. The top face of the three-axis load-cell is firmly connected to the vertical sliding system. The three-axis load-cell is capable of capturing the three orthogonal load components (x, y, and z) of up to 20 N in magnitude at the rubbing interface with an accuracy of 0.04 N in all the three directions. The whole vertical structure is equipped with a counterbalance load mechanism to flexibly adjust the normal force imposed onto the contacting interface in a tribocorrosion test. An arbitrary normal force can be attained by either adding or subtracting the amount of overhead dead weights and simultaneously reading the resulted normal force from the three-axis load-cell. The horizontal load-cell is also included to measure and record high-level shear forces (out of the capacity of the three-axis load-cell) when one decides to apply extremely high normal forces in a tribocorrosion test. The data from the load-cells are acquired using National Instruments module (NI; Model: NI 9237, USA) and are saved into a PC for further analysis. These data can be subsequently used for calculating the friction coefficients introduced into the interface.
The tribotester was equipped with a three-electrode set-up through which a constant potential (potentiostatic condition) can be applied to the interface and the resulted tribocorrosion current can be recorded as an indicator of the electrochemical reactions at the interface. According to the level of applied potential, either anodic or cathodic reactions would govern the electrochemical reactions. The three-electrode set-up was arranged by an Ag/AgCl reference electrode, a square Platinum plate as a counter electrode, and a working electrode (in this case; the metallic disk with an exposed surface area of 2.85 cm2). The working electrode is required to be electrochemically isolated from the rest of the system. To do so, a Polylactic acid (PLA) chamber was designed, and 3D printed. The inside walls of the chamber were then carefully treated using a Polyurethane adhesive (Sika, Switzerland) to avoid any possible leakage during tribocorrosion tests (and consequently, not producing reliable results). The three electrodes were linked to a potentiostat (MetrOhm; Model: PGSTAT 204, Switzerland) whose parameters can be adjusted using its own software (Nova). The potentiostat can apply a constant cathodic/anodic potential to the interface and record the tribocorrosion current data. These data can then be used to calculate the volume loss removed due to the electrochemical reactions.
2.2 Sample Preparation
Zirconia balls (ZrO2 ≥ 96%) and Ti-6Al-4 V (ASTM F136) disks were used in these experiments. The 10 mm thick Ti-6Al-4 V disks were cut from a bar with a diameter of 19.05 mm (Magellan Metals, USA). The zirconia balls (Shandong Ultraming Fine Ceramics, China) had a diameter of 9.525 mm. Table 1 lists the chemical compositions of the Ti-6Al-4 V alloy used in this study, according to the manufacturer’s material certificate. The metallic disks were prepared and polished using 120-, 240-, 400-, and 600-grit sandpapers. The disk’s lateral face was carefully masked using a Sealer Concentrate (Caswell, USA) to ensure that their possible unwanted electrochemical contributions to the experiments are avoided. This sealer is a fast-drying coating which firmly adheres to the target surface as a corrosion resistant layer and protects the underlying metal against corrosive environments. In this research, the first layer of the sealer was brushed onto the disks’ walls, and it was allowed to be cured for 30 min at room temperature. Then, the second coating layer was applied to ensure electrochemical isolation of the lateral faces. The masked disk would then be placed into the chamber’s seat, with its surroundings carefully sealed with silicone adhesive to prevent any solution’s possible leakage. The exposed circular surface of the disk was cleaned with Methanol to remove any possible contamination while preparing/seating the disk. The PBS solution (Sigma-Aldrich, USA) was used as the medium in this study.
2.3 Potentiodynamic and Tribocorrosion Tests
Before starting any tribocorrosion test, three potentiodynamic tests were conducted using three Ti-6Al-4 V disks. The applied potential to the interface was changed from − 1.5 V/VRef to 1 V/VRef with a constant sweep rate of 0.002 V/s. After identifying the corrosion potential of Ti-6Al-4 V, the tribocorrosion tests were planned to be conducted under potentiostatic conditions such that both the cathodic and anodic regions are included. These tests were conducted in three steps; in the first step, a cathodic potential of − 1.5 V/VRef was imposed onto the interface for 5 min to remove any existing oxide layer from the disk surface. In the second step, the interface was allowed to stabilise for 5 min under open circuit potential (OCP). Finally, in the third step, the constant potential (cathodic/anodic) was imposed onto the interface and the interface was then allowed to completely passivate. The stabilisation time under the imposed potential for the anodic and cathodic potentials were selected as 8 and 5 min, respectively. The stabilisation time was selected when the current (before starting the rubbing) decreased to very small magnitudes (less than 1e-6 A) with small fluctuations. After achieving the stability, the ball gripper was gently lowered to come into contact with the disk’s surface and the amount of dead weights was altered to adjust the level of normal force (contact pressure) accordingly. The normal forces of 3.5 N, 6 N, 10.8 N, and 17.5 N were chosen for this study which corresponded to maximum initial Hertzian contact pressures of 573.8 MPa, 686.8 MPa, 835.4 MPa, and 981.2 MPa, respectively. For ensuring that the stabilisation current is not influenced by the contact between the ball and disk, the set-up was kept motionless for more 2 min. After 2 min, the actuator was then activated to apply a nominal sliding distance of 4.8 mm for 1800 cycles with a sliding frequency of 1 Hz. All the tribocorrosion tests were repeated three times to ensure the reproducibility of the results. The resulted tribocorrosion current and forces were recorded, respectively, by the Nova and NI SignalExpress software environments for post analyses.
2.4 Surface Characterisations
After completing the tribocorrosion tests, the disks were cleaned with Methanol for surface characterisations including scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) (Inspect, Model: F50, USA), and hardness measurements (Struers; Model: DuraScan, Denmark). For the SEM, three wear tracks on the Ti-6Al-4 V disks and their zirconia counterparts were analysed. Sputter coating (EmiTech, Model: K575X) was used for the zirconia balls through which a 25 nm-thick Pt coating was applied onto their surface before mounting into the SEM chamber. All the wear tracks were then focussed with different magnifications under an accelerating voltage of 20 kV and a working distance of 10 mm. Point- and mapping-EDX were also carried out on the wear tracks to evaluate possible changes in the compositional elements and their distributions inside the wear tracks. The applied Vicker’s load was gradually increased from HV0.25 to HV10 to ensure that a valid Vicker’s indentation with well-defined diagonals is formed over the rough surface inside the wear tracks. Hence, the Vicker’s hardness measurements were conducted inside and outside the abraded regions consistently with HV10 and a dwell time of 3 s. For measuring the hardness, each wear track was tested for three times to ensure data repeatability.
2.5 Determination of Volume Loss and Tribological Constants
A roughness meter (Mitutoyo, Surftest; Model: SJ-410, Japan) was used to determine the total volume loss in this work. A low speed of 0.05 mm/s was chosen to capture fine irregularities (valleys and/or peaks) on the surface of the disk. For each wear track, three different lines (positioned at three different lengths of the track) were profiled alongside the track's width (ASTM- G133-05) based on the recommendations made in Ayerdi et al. [33]. The trapped area by each resulted profile was calculated, and then integrated alongside the length of the wear track to obtain the material volume loss. Therefore, three volume losses were obtained for each wear track which were subsequently averaged to determine the total volume loss of that wear track.
The rubbing currents measured by the potentiostat were substituted into Faraday’s law to determine the chemical volume loss. Within the anodic domain, the total rubbing current captured by the potentiostat constitutes of two main components resulted from dissolution and passivation phenomena which are called “corrosion- and “layer formation- “currents, respectively [34]. For a passive metal, the contribution of corrosion current into the total current is typically negligible within the anodic domain [10, 34, 35]; hence, the total current is assumed to be a representative of passivation only. Therefore, the calculated chemical losses represent the volume loss removed in an oxidised form (mechanically assisted corrosion), as defined and outlined in Cao and Mischler [35]. This calculation was performed using the test duration of 1,800 s, Faraday’s constant as 96,500 C/mol, and the oxide (TiO2) properties including an oxidation valence of 4, a density of 4.45 gr/cm3, and a molecular mass of 79.87 g/mol [36]. The oxide valence can vary with the potential level and pH of the environment. Based on the results published in [37], the main possible form of titanium oxide in the anodic potentials is TiO2; therefore, the valence number was assumed to be 4. Under the cathodic potentials selected and tested for this study, no oxide layer forms since the level of these potentials are lower than the corrosion potential of titanium [38]. However, corrosion of this alloy is highly possible. Within the framework of mechanistic approach [10, 39], the current from the anodic activities in the cathodic domain can be used to calculate the chemical loss due to dissolution. Having the chemical losses determined, the mechanical volume losses (the material removed from the interface in the form of metallic particles) were then obtained by subtracting the chemical losses from the total losses.
For finding the mechanical and electrochemical wear constants, Archard [40] and Landolt et al. [30] theories were used, respectively, as follows:
where \({K}_{m}, {\text{K}}_{\text{c}}\) are the tribological constants, \(\frac{{V}_{mech}}{s}\) the mechanical volume loss per each unit of sliding, s the overall sliding distance, FN the normal force, H the hardness, \(\frac{{V}_{chem}}{s}\) the chemical volume loss per each unit of sliding, and \({\text{Q}}_{\text{p}}\) is the charge density for passivation. These theories relate the volume loss to a linear function of mechanical and electrochemical parameters at a rubbing interface. For the passivation charge density in Eq. 2, the integration of the current density curve before rubbing was calculated for each level of imposed anodic potential.
3 Results
3.1 Potentiodynamic Polarisation Curve of Ti-6Al-4 V in PBS Solution
Potentiodynamic experiments resulted in an average corrosion potential of − 0.15 V/VRef, as illustrated in Fig. 2. It can be observed that the passivity stabilises the amount of current density emitted from the Ti-6Al-4 V disk after a potential of about 0 V/VRef up to 1 V/VRef. To include both the cathodic and anodic regions in the tribocorrosion tests, the candidate potentials for testing were accordingly selected as − 1.2 V/VRef and − 0.6 V/VRef for the cathodic region, and 0 V/VRef, 0.4 V/VRef, and 0.8 V/VRef for the anodic region.
3.2 Tribocorrosion Current Transients
The tribocorrosion current (after starting the rubbing) under an applied normal force of 17.5 N and a potential of 0.8 V/VRef is illustrated in Fig. 3a. One can see that the current level before the start of tribocorrosion is of decreasing pattern, which is due to the completion process of oxidation, and stabilises at very small values less than 1e-6 A. Starting the rubbing process after 600 s, the current ascends and descends sharply due to depassivation and the high rate of subsequent repassivation. From the start of rubbing at 600 s to the end of the test at 2400 s, the mean current is constant at a value far greater than the stabilised passivation current (around 100 times). The current then returns to the small magnitudes less than 1e-6 A once the rubbing stops. The mean rubbing current (Imean) was determined by subtracting the average of the currents before and after the rubbing process from the mean of the recorded currents. This subtraction excludes the contribution of disk's un-rubbed area into the total current. The mean current for various levels of applied anodic potentials and normal forces studied in this work are listed in Table 2. One can see that the mean anodic current increases proportionally with increasing the normal force. The results of this table are also exhibited in Fig. 4 with their error bars at each level of imposed potential. With an increase in the normal load from 3.5 to 17.5 N, it can be observed that the maximum increase in the mean anodic current is 88 µA, 123 µA, and 144 µA at the potentials of 0, 0.4, and 0.8 V/VRef, respectively. All the anodic current data are in the range of 4.60e−5–219e−4 A, and they increase with both the normal force and potential level.
Under the cathodic potentials, the current stabilised at a very small negative current less than − 1e−6 A, as illustrated in Fig. 3b, c. Starting the rubbing at a potential of − 0.6 V/VRef, it was observed that current moved to the anodic region with small magnitudes (Fig. 3b). The current returned to the stabilised level once the rubbing was stopped. The mean current under a potential of − 0.6 V/VRef was determined by subtracting the mean of current during rubbing from the stabilised current before and after the rubbing test. Under the normal forces of 17.5, 10.8, 6, and 3.5 N, the induced currents were, respectively, 10.2e−6, 7.3e−6, 4.6e−6, and 1.7e−6 A. Under the cathodic potential of − 1.2 V/VRef, the current dropped to more negative values by the start of the rubbing process (Fig. 3c). The resulted mean values of cathodic currents under the normal forces of 17.5, 10.8, 6, and 3.5 N were measured as 2.36e−4, 1.48e−4, 7.36e−5, and 4.73e−5 A, respectively. It was also observed that the reproducibility of the current results was satisfactory with maximum deviation of 6% from the average value.
3.3 Friction and Wear
The three-axis load-cell used in the tribotester measured both the normal and shear forces during sliding. Figure 5 shows the recorded forces for the two directions under an imposed potential of 0.8 V/VRef and a normal force of 17.5 N for five cycles of rubbing as a representation of the whole tribocorrosion experiment. Positive and negative values of the recorded shear force profile show the forward and backward motions of a rubbing cycle, respectively. The coefficient of friction (CoF) was determined by dividing the measured shear force by the corresponding normal force. CoF did not change during the tribocorrosion cycles and it showed some variations among the 3 test repetitions (with some interceptions for various potentials at each normal force). Therefore, additional experiments were planned and conducted to ensure that the variations in CoFs were well captured. These tests were conducted for 200 cycles with five repetitions for each testing condition (totally 100 tests). The friction coefficient was found to be independent of the imposed potential; however, the mean value of CoF was observed to decrease by an increase in the applied normal force (Fig. 6). As shown, the mean CoF changes from 0.84 to 0.44 with increasing the normal force from 3.5 to 17.5 N and the variation of CoFs is more noticeable for the test cases conducted under lower normal forces specially the ones at 3.5 N.
3.4 Material Volume Loss and Hardness Measurements
Figure 7 exhibits an example of tribocorrosion depth captured over the wear width for an imposed potential of 0.8 V/VRef under various normal forces. One can see that the maximun wear depth increaes with the applied normal force. The maximum wear depth was around 11.3, 14.1, 35.4, and 40 µm for the tests conducted at 3.5, 6, 10.8, and 17.5 N, respectively. In addition, the specific maximum wear depth (maximum wear depth divided by the corresponding normal force) is maximised for the test at 10.8 N. The acccumulation of solid particles is confirmed at the edge of the wear tracks which increaes with the level of applied normal force.
In the cathodic domain, the obtained currents were used to determine the corresponding chemical losses due to corrosion/dissolution within the framework of mechanistic approach. This chemical loss was responsible for around maximum 2% of the total loss. Therefore, this contribution was neglected in this study which is also consistent with the assumption made by Namus et al. [10]. In addition, there is a controversy in the literature that under certain circumstances, the total loss in the cathodic domain cannot be assumed as purely mechanical since the wear characteristics of the interface are altered by hydrogen segregation and interface embrittlement [41]. However, it is still common to assume this total loss as being mechanical, as detailed and used by other researchers in the literature [10, 13, 29, 35, 42]. Therefore, taking into account this concept, this study assumed the total loss in the cathodic domain as mechanical. The results of mechanical, chemical and total volume losses are accordingly listed in Table 3. The average total loss ranges from 0.0144 to 0.125 mm3 and overall increases with both the normal force and potential (with some interceptions especially under normal forces of 3.5 and 6 N). The average mechanical and chemical losses change in the ranges of 0.0139–0.104 and 0.00428–0.0204 mm3, respectively. The chemical losses follow direct relationships with the normal force and potential. This direct relationship is consistent with the predictions of the tribocorrosion theories [28, 30] wherein with an increase in either/both the normal force and potential, the tribocorrosion current (and thus, the chemical wear) increases. The mechanical loss increases with the normal force (with some interceptions at normal forces of 3.5 and 6 N) which is also consistent with Archard’s law [40] where higher normal forces result in more mechanical wear at an interface. The mechanical loss in the cathodic region increases with an increase in the potential; however, in the anodic region, the situation is rather different such that the mechanical loss decreases with an increase in the potential level. In the anodic region, the electrochemical reactions are responsible for maximum 36% of the total volume loss (Fig. 8). Overall, the proportional contribution by chemical wear increases with an increase in the imposed potential (higher oxidation rates). Interestingly, the proportional role of chemical wear is maximum for the test cases conducted under the normal force of 6 N. According to the criterion proposed by Stack and Abdulrahman [43] which has been used for Ti-6Al-4 V in [12, 16] and also the presented data in Fig. 8, the degradation mechanism under the anodic potentials at all normal force levels is classified as “wear-corrosion”.
The results of microhardness measurements (Table 3) indicated no significant changes in the hardness after tribocorrosion under different levels of applied normal force and potential. The tests conducted under a normal force with various potentials showed approximately similar hardness values. Overall, a mean hardness value of 380 HV can be considered for the tribocorroded Ti-6Al-4 V under different testing conditions which is close to the hardness of un-rubbed regions as 368 HV (3.26% difference). The results of Table 3 together with the material constants were then used to determine the tribological constants of Ti-6Al-4 V using Eqs. 1 and 2. The results of such analysis are illustrated in Fig. 9. The charge density for anodic potentials of 0 V/VRef, 0.4 V/VRef, and 0.8 V/VRef were obtained as 2.27, 2.32, and 2.54 mC/cm2, respectively. Given the overall sliding distance for 1,800 cycles was 17,280 mm, one can obtain the mechanical and chemical volume losses as linear functions of \(\frac{{F}_{N}}{H}\) and \({Q}_{p}{\left({F}_{N}/H\right)}^{0.5}\) with slopes of 1.24e−4 (Km) and 2.43e−7 (Kc), respectively. These slopes can now be used as indicators to calculate the mechanical and chemical volume losses from Eqs. 1 and 2 for an arbitrary combination of applied normal force and imposed anodic/cathodic potential.
3.5 Surface Characterisation
SEM was carried out on three wear tracks with potentials and normal forces of 0.8 V/VRef and 17.5 N, 0.8 V/VRef and 3.5 N, and -1.2 V/VRef and 17.5 N (Fig. 10). These wear tracks were strategically selected to find possible influences of the normal force and potential. SEM results confirmed the enlargement in the wear tracks with an increase in potential and/or normal force. Parallel rubbing scratches exist continuously in alignment with the sliding direction all along the wear tracks. These scratches seem to become deeper and also narrower while moving from the edge of the track to the mid-width region which may be related to the size and shape of the wear particles in these regions. As Fig. 10 shows, the ceramic ball counterparts remained almost undamaged and some material, in the form of wear particles from the disk, are observed on the ball surface. The presence of scratches with different depths and also having the ceramic counterparts as almost undamaged, it could be implied that the wear track on Ti-6Al-4 V disks is enlarged by the action of the third-body wear particles. Small surface cracks (material's tearing out) inside the wear tracks can be detected; however, no pitting was observed inside the wear tracks for the tested cases in this study. The accumulation of solid particles inside the wear tracks are also detectable. Point-analysis EDX inside the wear tracks showed no significant changes in the alloy composition compared to the untested alloy except minor Potassium (K), Sodium (Na), Phosphorous (P), Oxygen (O), and Chlorine (Cl), and also very small amount of Zirconium (Zr) (indicating that the zirconia ball remained almost undamaged) debris inside the wear tracks. EDX mapping technique for small areas (20 × 20 µm2) inside the wear tracks showed regional accumulations/decumulations of the elements; however, in the case of the Ti-6Al-4 V disk, the main elements of the substrate show a more unform distributions.
4 Discussion
The present study investigated the mechanical and chemical wear behaviour in the tribocorrosion of Ti-6Al-4 V immersed into PBS solution under different levels of normal force in both the anodic and cathodic regions. The mechanical and electrochemical reactions at the contacting interface of Ti-6Al-4 V with zirconia were measured and compared to provide a basis for extracting the tribological constants of this alloy for relevant applications. On the mechanical aspect, CoF increased with decreasing the normal force which is consistent with some findings in the literature [6]. One possible explanation for the reduction in CoF with an increase in the normal force is a decrease in the size and angularity of third-body wear particles and therefore, possible smoothening of the interface as reported by Mardare et al. [6]. Having smaller and smoother wear particles in the wear track, the rubbing of the counterparts is facilitated resulting in a decrease in CoF. Micro-hardness after the tribocorrosion tests was similar to that of un-rubbed areas. This is partly consistent with findings in [44] but disagrees with the results reported in Yazdi et al. [29]. This disagreement may be due to the levels of normal force used in their study (3–30 N [29] vs 3.5–17.5 N in this study), and balls’ diameter and material (alumina-∅5 mm [29] vs. zirconia-∅9.525 mm in this study) which finally results in different contact pressures and possible plastic deformations at the contacting interface. It may be concluded that the higher the normal force the higher the changes in surface hardness and possible sub-surface grain refinements. The negligible change in hardness may signify no significant contribution of surface hardening into changing the ductility of the contacting interface; hence, the total material loss [29]. Another possible reason for the disagreement is the potential used in this study versus the OCP condition used in Yazdi et al. [29]. Anodic potentials can change the stability and the mechanical properties of the oxide layer formed over substrate [12] and cathodic potentials may increase the hardness of the substrate by hydrogen embrittlement process [41]. Therefore, a possible change in the hardness of the wear tracks after experiencing the tribocorrosion cycles is entailed by changing the applied potential.
For the electrochemical reactions at the interface, the mean current increased with an increase in the normal force and the potentials. The mean current due to repassivation remained constant throughout the course of the tribocorrosion tests which agrees with the results in the literature [4] and is somewhat different from what observed in [16]. The change in the mean current can be related to the enlargement of the contacting area with the number of tribocorrosion cycles. Having both the contacting area enlarged with the tribocorrosion cycles and also given the increase in the current density with an increase in the abrasion rate [28, 30], the constant mean current implies that the mean abrasion rate of the oxide layer decreases with the tribocorrosion cycles. Rubbing passivation current due to the potential evolution in the OCP condition (so-called as “cathodic excursion”) has been reported in the literature [35, 45]. The level of cathodic excursion in an industrial problem (and even in ball-on-disk configurations [29]) depends on various parameters such as the applied loading (magnitudes and directions), material combinations in contact, contact geometry, and the solution type and its pH [32]. Therefore, the cathodic excursion needs to be measured in a series of separate application-oriented experiments. The anodic current under the OCP condition due to the cathodic excursion and the resultant galvanic couple between the un-rubbed and rubbed areas can be obtained using the coupling model developed by Vieira et al. [35, 45] and it can be also measured using zero-resistance ammetry (ZRA) technique proposed by Espallargas et al. [46]. These two techniques were applied on Ti-6Al-4 V alloy by Licausi et al. [47] and the experimental results were satisfactorily consistent with those from the model developed by Vieira et al. [45].
The chemical wear accounted for up to 36% of the total tribocorrosive damage. Under a constant level of imposed anodic potential, the role of chemical wear in the total wear (proportional chemical loss) was maximum for the tests conducted at 6 N followed by the tests at 3.5 N, 10.8 N, and 17.5 N, respectively. This suggests that the normal force of 6 N introduces a more severe damage into the oxide layer which follows the electrochemical pattern observed for Ti-6Al-4 V-Ti-6Al-4 V pin-disk interfaces in Swaminathan and Gilbert [48]. For the tests under a constant normal force, the role of chemical wear in the anodic region increased with the imposed potential which originates from a higher oxidation rate (and thus, current) [28]. In the anodic region, the mechanical loss decreased with an increase in the potential which is consistent with some findings on Ti-6Al-4 V in the literature [12]. This reduction is mainly attributed to the higher proportional role of the chemical wear in the total wear, as observed in Fig. 8. The amount of mechanical loss under the cathodic domain was observed to increase with an increase in the imposed potential. This increase might be caused by the shear cutting process at the interface because of the larger sizes of the detached particles. The mechanical loss was observed to increase with the normal force from 6 to 17.5 N which is consistent with the predictions of Archard’s law [40]. These data however had some interceptions at the normal forces of 3.5 and 6 N which introduce lower contact pressures to the interface. This may be justified by the angularity of the wear particles which should be more smoothened out under the normal force of 6 N compared to that under 3.5 N. The change in the total loss with normal force was roughly similar to that of the mechanical wear. Overall, the total loss in the anodic region at higher normal forces increased with the applied potential possibly due to the formation of a more stable oxide layer. The more stable the oxide layer, the more the dislocations piled up below the surface. Therefore, the interface becomes more prone to crack initiation and rupture (known as “Roscoe effect” [49, 50]) leading to higher total losses. However, the total losses in the anodic region under the normal force of 3.5 N decreased with an increase in the potential level. This may be attributed to the stability of oxide layer under higher anodic potentials, as observed by Runa et al. [12] which may subsequently protect the interface against lower levels of normal force (in this study, 3.5 N). Under cathodic potentials considered in this study (− 0.6 and − 1.2 V/VRef), the titanium is not in its immunity region and the corrosion of metal is possible. However, the passivation does not seem to occur because the cathodic potentials are lower than the corrosion potential of titanium [38]. Therefore, the increase in the material loss under cathodic potentials may be attributed to the contribution of the corrosion to the material loss. Under the cathodic potentials, the current stabilised at small magnitudes with negligible fluctuations which implies no electrochemical evolutions at the interface before the start of rubbing. Under the cathodic potential of − 1.2 V/VRef, the current remained in the negative domain and dropped to more negative currents once the rubbing was started. This negative drop may be due to the oxygen reduction process due to the solution motions and subsequent oxygen absorption at the interface, as discussed by Ren et al. [13] for Ti-6Al-4 V at a potential of − 1 V/V Ag/AgCl. Another possible reason for this drop is hydrogen segregation at the interface [13, 41, 51] which can subsequently reduce the ductility of the interface and induce hydrogen embrittlement. Under the cathodic potential of − 0.6 V/VRef and by starting the rubbing, the current moved to the anodic domain with small magnitudes. This current is possibly due to the dissolution of the substrate ions into the solution. The influence of the above-mentioned mechanisms on the mechanical wear is more significant at lower normal forces in Fig. 9 where the mechanical losses of the cathodic potentials are close (and in some cases higher) than those measured in the anodic domain. The influence of hydrogen embrittlement seems not to predominate the interface for the tests included in this study since the material losses became comparatively greater while moving towards less cathodic potentials. This has been also reported by Ren et al. [13] where under a cathodic potential of − 1 V/VRef, the immersion of Ti-6Al-4 V into deionised water entailed nearly equal material losses with those observed in seawater. Hence, the negative drop in the cathodic currents may be more controlled by the oxygen absorption at the interface, as reported by Ren et al. [13]. Since the passivation does not occur under both the cathodic potentials considered in this study, the anodic activity of the interface is attributed to the dissolution phenomenon. The contribution of this anodic activity to the total loss was negligible; and therefore, the total loss under the cathodic potentials was assumed to be mechanical, as in Namus et al. [10]. Overall, the mechanical loss data here were described by a linear pattern with a coefficient of determination of R2 = 0.96 (Fig. 9). The interactive influence of all the above-mentioned reasons may explain the interceptions of the measured CoFs at different potentials under a constant level of normal force.
The results of volume loss obtained in this study were used for calibrating the constants of a well-known tribocorrosion theory and Archard’s law for future analyses in relevant applications such as biomedical implants. The present study has a number of limitations. The results of this study are limited to the testing parameters and conditions including the PBS solution employed in this work. However, the tribocorrosion behaviour of Ti-6Al-4 V can be also tested and explored in different chemical solutions with various pHs (and also possible inclusions of complexities associated with real applications), different sliding modes (gross- and partial-slip), longer testing times, and different sliding frequencies to be usable in various applications. Furthermore, this study was performed at room temperature which is fairly low and represents some applications where the temperature of the working environment does not affect the passivation kinetics as discussed in detail by Namus et al. [10]. However, a range of applications experience high temperatures wherein the induced temperature is capable of changing the oxide layer stability, passivation rate, and also mechanical ductility of the interface; thereby, the total loss and the interrelations of the mechanical and electrochemical reactions at the interface [52]. As for the post analyses, hardness measurements should be conducted with nanoindentations to find the hardness values as localised as possible over the surface of wear tracks. This could be challenging specially where the scratches inside a wear track are of high roughness. The characterisation could be also extended to include atomic force microscopy (AFM) and focus ion beam (FIB) analysis to explore the adhesion of wear particles and possible phase changes inside and outside the wear tracks, and possible sub-surface grain refinements, respectively. Furthermore, the tribological constants were extracted within the framework of a two-body contact system where the influence of the hydrodynamic film, produced by the captivated solution in between the contacting asperities, are not incorporated into their fundamental formulations. The application of the so-called “lubricated tribocorrosion models” [53] requires extensive experimental efforts to strategically measure and obtain the influence of the hydrodynamic film on the effective normal force at the interface [35]. The tribocorrosion behaviour of Ti-6Al-4 V and hence material loss is also altered with different treatments [54,55,56]. These were beyond the scope of the present study. Nevertheless, within the limitations of this study, the data in this work can now be combined with nonlinear numerical analyses (such as finite element) for gaining an insight into the early stages of the tribocorrosion behaviour of Ti-6Al-4 V alloy used in real applications including the biomedical implants.
5 Conclusions
In this paper, the tribocorrosion behaviour of Ti-6Al-4 V alloy exposed to PBS was investigated under various levels of normal force in both the cathodic and anodic regions when in contact with zirconia. The integrated data obtained in this study was used to calibrate the tribological constants of this alloy for evaluating the mechanical and chemical wear loss processes. The main conclusions of the present study are as follows:
-
(1)
The chemical wear mechanism played a major role up to 36% of the total material loss. The relationship of the proportional chemical wear with normal force was neither direct nor reverse such that a mid-level normal force (6 N) resulted in the maximum proportional chemical loss.
-
(2)
The mechanical wear loss increased with the imposed potential in the cathodic region possibly due to dissolution procedures involved at the interface. Under anodic domain, the mechanical loss decreased with an increase in the potential due to higher proportional role played by the chemical wear in a higher anodic potential.
-
(3)
Increasing the imposed potential resulted in an increase in the total loss. This is possibly due to the shape and size of the detached particles. Higher potentials may increase the size and angularity of the wear particles at the interface and consequently, higher material loss rate due to the shear cutting process. Higher potentials also increase the oxidation rates which may result in an increase in the material loss. Overall, the influence of both mechanical and chemical wear mechanisms (and their influential parameters) helped to equip a tribocorrosion theory with its related constants to be used in future for simulations and further analyses in relevant applications to obtain a better insight into the tribocorrosion behaviour of Ti-6Al-4 V.
References
Long, M., Rack, H.J.: Titanium alloys in total joint replacement—a materials science perspective. Biomaterials (1998). https://doi.org/10.1016/S0142-9612(97)00146-4
Molinari, A., Straffelini, G., Tesi, B., Bacci, T.: Dry sliding wear mechanisms of the Ti6Al4V alloy. Wear (1997). https://doi.org/10.1016/S0043-1648(96)07454-6
Ganesh, B.K.C., Ramanaih, N., Chandrasekhar Rao, P.V.: Dry sliding wear behavior of Ti–6Al–4V implant alloy subjected to various surface treatments. Trans. Indian Inst. Met. (2012). https://doi.org/10.1007/s12666-012-0147-4
Licausi, M.P., Igual Muñoz, A., Amigó Borrás, V.: Influence of the fabrication process and fluoride content on the tribocorrosion behaviour of Ti6Al4V biomedical alloy in artificial saliva. J. Mech. Behav. Biomed. Mater. (2013). https://doi.org/10.1016/j.jmbbm.2013.01.019
Hammood, A.S., Thair, L., Altawaly, H.D., Parvin, N.: Tribocorrosion behaviour of Ti–6Al–4V alloy in biomedical implants: effects of applied load and surface roughness on material degradation. J. Bio. Tribo Corros. (2019). https://doi.org/10.1007/s40735-019-0277-x
Mardare, E., Benea, L., Celis, J.-P.: Importance of applied normal loads on the tribocorrosion behaviour of Ti-6Al-4V alloy in bio-simulated environment. Optoelectron. Adv. Mater. Rapid Commun. 6, 474–478 (2012)
Pejaković, V., Totolin, V., Ripoll, M.R.: Tribocorrosion behaviour of Ti6Al4V in artificial seawater at low contact pressures. Tribol. Int. (2018). https://doi.org/10.1016/j.triboint.2017.10.025
Dong, H., Bell, T.: Tribological behaviour of alumina sliding against Ti6Al4V in unlubricated contact. Wear (1999). https://doi.org/10.1016/S0043-1648(98)00407-4
Komotori, J., Hisamori, N., Ohmori, Y.: The corrosion/wear mechanisms of Ti–6Al–4V alloy for different scratching rates. Wear (2007). https://doi.org/10.1016/j.wear.2006.11.025
Namus, R., Nutter, J., Qi, J., Rainforth, W.M.: Sliding speed influence on the tribo-corrosion behaviour of Ti6Al4V alloy in simulated body fluid. Tribol. Int. (2021). https://doi.org/10.1016/j.triboint.2021.107023
Philip, J.T., Mathew, J., Kuriachen, B.: Tribology of Ti6Al4V: a review. Friction (2019). https://doi.org/10.1007/s40544-019-0338-7
Runa, M.J., Mathew, M.T., Rocha, L.A.: Tribocorrosion response of the Ti6Al4V alloys commonly used in femoral stems. Tribol. Int. (2013). https://doi.org/10.1016/j.triboint.2013.09.022
Ren, P., Meng, H., Xia, Q., Zhu, Z., He, M.: Influence of seawater depth and electrode potential on the tribocorrosion of Ti6Al4V alloy under the simulated deep-sea environment by in-situ electrochemical technique. Corros. Sci. (2021). https://doi.org/10.1016/j.corsci.2020.109185
Licausi, M.-P., Muñoz, A.I., Borrás, V.A.: Tribocorrosion mechanisms of Ti6Al4V biomedical alloys in artificial saliva with different pHs. J. Phys. D (2013). https://doi.org/10.1088/0022-3727/46/40/404003
Çaha, I., Alves, A.C., Chirico, C., Tsipas, S.A., Rodrigues, I.R., Pinto, A.M.P., Grandini, C.R., Rocha, L.A., Gordo, E., Toptan, F.: Interactions between wear and corrosion on cast and sintered Ti-12Nb alloy in comparison with the commercial Ti-6Al-4V alloy. Corros. Sci. (2020). https://doi.org/10.1016/j.corsci.2020.108925
Royhman, D., Yuan, J., Shokuhfar, T., Takoudis, C., Sukotjo, C., Mathew, M.: Tribocorrosive behaviour of commonly used temporomandibular implants in a synovial fluid-like environment: Ti–6Al–4V and CoCrMo. J. Phys. D (2013). https://doi.org/10.1088/0022-3727/46/40/404002
Hacisalihoglu, I., Samancioglu, A., Yildiz, F., Purcek, G., Alsaran, A.: Tribocorrosion properties of different type titanium alloys in simulated body fluid. Wear (2015). https://doi.org/10.1016/j.wear.2014.12.017
Feyzi, M., Fallahnezhad, K., Taylor, M., Hashemi, R.: A review on the finite element simulation of fretting wear and corrosion in the taper junction of hip replacement implants. Comput. Biol. Med. (2021). https://doi.org/10.1016/j.compbiomed.2020.104196
Feyzi, M., Fallahnezhad, K., Taylor, M., Hashemi, R.: The mechanics of head-neck taper junctions: what do we know from finite element analysis? J. Mech. Behav. Biomed. Mater. (2021). https://doi.org/10.1016/j.jmbbm.2021.104338
Fallahnezhad, K., Oskouei, R.H., Badnava, H., Taylor, M.: An adaptive finite element simulation of fretting wear damage at the head-neck taper junction of total hip replacement: the role of taper angle mismatch. J. Mech. Behav. Biomed. Mater. (2017). https://doi.org/10.1016/j.jmbbm.2017.07.003
Hussenbocus, S., Kosuge, D., Solomon, L.B., Howie, D.W., Oskouei, R.H.: Head-neck taper corrosion in hip arthroplasty. Biomed. Res. Int. (2015). https://doi.org/10.1155/2015/758123
Fallahnezhad, K., Oskouei, R.H., Taylor, M.: Development of a fretting corrosion model for metallic interfaces using adaptive finite element analysis. Finite Elem. Anal. Des. (2018). https://doi.org/10.1016/j.finel.2018.05.004
Dimah, M.K., Albeza, F.D., Borrás, V.A., Muñoz, A.I.: Study of the biotribocorrosion behaviour of titanium biomedical alloys in simulated body fluids by electrochemical techniques. Wear (2012). https://doi.org/10.1016/j.wear.2012.04.014
Gopal, V., Manivasagam, G.: Wear—corrosion synergistic effect on Ti–6Al–4V alloy in H2O2 and albumin environment. J. Alloy. Compd. (2020). https://doi.org/10.1016/j.jallcom.2020.154539
Chao, C., Lin, L., Macdonald, D.: A point defect model for anodic passive films: I film growth kinetics. J. Electrochem. Soc. (1981). https://doi.org/10.1149/1.2127591
Bartolomeu, F., Buciumeanu, M., Pinto, E., Alves, N., Silva, F., Carvalho, O., Miranda, G.: Wear behavior of Ti6Al4V biomedical alloys processed by selective laser melting, hot pressing and conventional casting. Trans. Nonferrous Metals Soc. China (2017). https://doi.org/10.1016/S1003-6326(17)60060-8
Buciumeanu, M., Bagheri, A., Shamsaei, N., Thompson, S., Silva, F., Henriques, B.: Tribocorrosion behavior of additive manufactured Ti-6Al-4V biomedical alloy. Tribol. Int. (2018). https://doi.org/10.1016/j.triboint.2017.11.032
Mischler, S., Debaud, S., Landolt, D.: Wear-accelerated corrosion of passive metals in tribocorrosion systems. J. Electrochem. Soc. (1998). https://doi.org/10.1149/1.1838341
Yazdi, R., Ghasemi, H., Abedini, M., Wang, C., Neville, A.: Tribocorrosion behaviour of Ti6Al4V under various normal loads in phosphate buffered saline solution. Trans. Nonferrous Metals Soc. China (2020). https://doi.org/10.1016/S1003-6326(20)65297-9
Landolt, D., Mischler, S., Stemp, M.: Electrochemical methods in tribocorrosion: a critical appraisal. Electrochim. Acta (2001). https://doi.org/10.1016/S0013-4686(01)00679-X
Lee, H., Mall, S., Allen, W.Y.: Fretting fatigue behavior of shot-peened Ti–6Al–4V under seawater environment. Mater. Sci. Eng. A (2006). https://doi.org/10.1016/j.msea.2006.01.031
Fallahnezhad, K., Feyzi, M., Ghadirinejad, K., Hashemi, R., Taylor, M.: Finite element based simulation of tribocorrosion at the head-neck junction of hip implants. Tribol. Int. (2022). https://doi.org/10.1016/j.triboint.2021.107284
Ayerdi, J.J., Aginagalde, A., Llavori, I., Bonse, J., Spaltmann, D., Zabala, A.: Ball-on-flat linear reciprocating tests: critical assessment of wear volume determination methods and suggested improvements for ASTM D7755 standard. Wear (2021). https://doi.org/10.1016/j.wear.2021.203620
Vetter, K., Gorn, F.: Kinetics of layer formation and corrosion processes of passive iron in acid solutions. Electrochim. Acta (1973). https://doi.org/10.1016/0013-4686(73)80036-2
Cao, S., Mischler, S.: Modeling tribocorrosion of passive metals–a review. Curr. Opin. Solid State Mater. Sci. (2018). https://doi.org/10.1016/j.cossms.2018.06.001
Swaminathan, V., Gilbert, J.L.: Potential and frequency effects on fretting corrosion of Ti6Al4V and CoCrMo surfaces. J. Biomed. Mater. Res. A (2013). https://doi.org/10.1002/jbm.a.34564
Bhola, S.M., Mishra, B.: Effect of pH on the electrochemical properties of oxides formed over β–Ti-15Mo and mixed Ti-6Al-4V alloys. Int. J. Electrochem. Sci 8, 7075–7087 (2013)
Gudić, S., Vrsalović, L., Kvrgić, D., Nagode, A.: Electrochemical behaviour of Ti and Ti-6Al-4V alloy in phosphate buffered saline solution. Materials (2021). https://doi.org/10.3390/ma14247495
Munoz, A.I., Espallargas, N., Mischler, S.: Tribocorrosion. Springer, Cham (2020)
Archard, J.: Contact and rubbing of flat surfaces. J. Appl. Phys. (1953). https://doi.org/10.1063/1.1721448
Jiang, X., Li, S., Duan, C., Li, M.: A study of the corrosive wear of Ti-6Al-4V in acidic medium. Wear (1989). https://doi.org/10.1016/0043-1648(89)90266-4
Dong, M., Zhu, Y., Wang, C., Shan, L., Li, J.: Structure and tribocorrosion properties of duplex treatment coatings of TiSiCN/nitride on Ti6Al4V alloy. Ceramics Int. (2019). https://doi.org/10.1016/j.ceramint.2019.03.180
Stack, M., Abdulrahman, G.: Mapping erosion-corrosion of carbon steel in oil exploration conditions: some new approaches to characterizing mechanisms and synergies. Tribol. Int. (2010). https://doi.org/10.1016/j.triboint.2010.01.005
Brończyk, A., Kowalewski, P., Samoraj, M.: Tribocorrosion behaviour of Ti6Al4V and AISI 316L in simulated normal and inflammatory conditions. Wear (2019). https://doi.org/10.1016/j.wear.2019.202966
Vieira, A.C., Rocha, L.A., Papageorgiou, N., Mischler, S.: Mechanical and electrochemical deterioration mechanisms in the tribocorrosion of Al alloys in NaCl and in NaNO3 solutions. Corros. Sci. (2012). https://doi.org/10.1016/j.corsci.2011.08.041
Espallargas, N., Johnsen, R., Torres, C., Muñoz, A.I.: A new experimental technique for quantifying the galvanic coupling effects on stainless steel during tribocorrosion under equilibrium conditions. Wear (2013). https://doi.org/10.1016/j.wear.2013.08.026
Licausi, M.-P., Muñoz, A.I., Borrás, V.A., Espallargas, N.: Tribocorrosion mechanisms of Ti6Al4V in artificial saliva by zero-resistance ammetry (ZRA) technique. J. Bio. Tribo Corros. (2015). https://doi.org/10.1007/s40735-015-0008-x
Swaminathan, V., Gilbert, J.L.: Fretting corrosion of CoCrMo and Ti6Al4V interfaces. Biomaterials (2012). https://doi.org/10.1016/j.biomaterials.2012.04.015
Buckley, D.H.: Surface effects in adhesion, friction, wear, and lubrication. Elsevier, Hoboken (1981)
Barril, S., Mischler, S., Landolt, D.: Triboelectrochemical investigation of the friction and wear behaviour of TiN coatings in a neutral solution. Tribol. Int. (2001). https://doi.org/10.1016/S0301-679X(01)00052-4
Sun, Y., Bailey, R.: Effect of applied cathodic potential on friction and wear behavior of CoCrMo alloy in NaCl solution. Lubricants (2020). https://doi.org/10.3390/lubricants8110101
Wang, L., Zhang, Q., Li, X., Cui, X., Wang, S.: Severe-to-mild wear transition of titanium alloys as a function of temperature. Tribol. Lett. (2014). https://doi.org/10.1007/s11249-013-0289-5
Cao, S., Guadalupe Maldonado, S., Mischler, S.: Tribocorrosion of passive metals in the mixed lubrication regime: theoretical model and application to metal-on-metal artificial hip joints. Wear (2015). https://doi.org/10.1016/j.wear.2014.12.003
Manhabosco, T., Müller, I.: Tribocorrosion of diamond-like carbon deposited on Ti6Al4V. Tribol. Lett. (2009). https://doi.org/10.1007/s11249-009-9417-7
Allen, C., Bloyce, A., Bell, T.: The effect of ion implantation on the sliding wear behaviour of ultrahigh molecular weight polyethylene against an oxidised titanium alloy Ti-6Al-4V. Tribol. Lett. (1995). https://doi.org/10.1007/BF00157973
Cvijović-Alagić, I., Cvijović, Z., Mitrović, S., Rakin, M., Veljović, Đ, Babić, M.: Tribological behaviour of orthopaedic Ti-13Nb-13Zr and Ti-6Al-4V alloys. Tribol. Lett. (2010). https://doi.org/10.1007/s11249-010-9639-8
Acknowledgements
The authors would like to acknowledge the support through Flinders International Postgraduate Research Scholarship for Mr. Mohsen Feyzi's PhD; and ARC Centre for Medical Implant Technologies for the funding of Dr. Khosro Fallahnezhad’s position. The authors also would like to acknowledge the facilities and technical assistance of Microscopy Australia and the Australian National Fabrication Facility (ANFF) under the National Collaborative Research Infrastructure Strategy, at the South Australian Regional Facility, Flinders Microscopy and Microanalysis, Flinders University.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feyzi, M., Fallahnezhad, K., Taylor, M. et al. The Tribocorrosion Behaviour of Ti-6Al-4 V Alloy: The Role of Both Normal Force and Electrochemical Potential. Tribol Lett 70, 83 (2022). https://doi.org/10.1007/s11249-022-01624-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-022-01624-0