Abstract
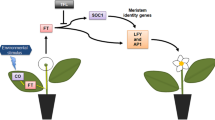
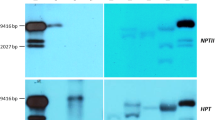
Transgenic ‘Duncan’ grapefruit (Citrus paradisi Macf.) and ‘Valencia’ sweet orange (Citrus sinensis [L.] Osbeck) plants ectopically expressing C. sinensis (cv. Washington navel orange) APETALA1 (CsAP1) or LEAFY (CsLFY) genes under control of the Arabidopsis thaliana stress-inducible promoter AtRD29A flowered under non-inductive (warm temperature, well-watered) greenhouse conditions, whereas their wild-type (WT) counterparts did not. The transgenic plants that flowered exhibited no altered morphological features, except the lack of thorns characteristic of juvenile WT plants. The most precocious T0 line, ‘Duncan’ grapefruit (Dun134-3) expressing the CsAP1 gene, flowered and fruited when it was 4.5 years old and the T1 siblings from this line flowered and fruited when they were just over 18 months old. In contrast, T1 seedlings from three lines of ‘Duncan’ grapefruit expressing the CsLFY gene flowered within 3 months after germination, but were unable to support fruit development. Transcript levels of corresponding transgenes in leaves were not correlated with earliness of flowering. To further study the activity of AtRD29A, leaves from three ‘Carrizo’ citrange (C. sinensis × Poncirus trifoliata) rootstock seedlings transformed with the green fluorescent protein (GFP) gene under regulation of the AtRD29A promoter were subjected to drought stress or well-watered conditions. Expression of GFP was not stress-dependent, consistent with the observation of flowering of CsAP1 and CsLFY transgenic plants under non-inductive conditions. Taken together, the results suggest that AtRD29A is constitutively expressed in a citrus background. Despite the loss of control over flowering time, transgenic citrus lines ectopically expressing C. sinensis AP1 or LFY genes under control of the A. thaliana RD29A promoter exhibit precocious flowering, fruit development and viable transgenic seed formation. These transformed lines can be useful tools to reduce the time between generations to accelerate breeding.





Similar content being viewed by others
References
Aleza P, Jimirez J, Ollitrault P, Navarro L (2008) Selection of spontaneous autotetraploid plants from Citrus polyembryonic cultivars. In: Prohens J, Badenes ML (eds) Modern variety breeding for present and future needs. Universidad Politécnica de Valencia, Valencia
Behnman B, Kikuchi A, Celebi-Toprak F, Yamanaka S, Kasuga M, Yamaguchi-Shinozaki K, Watanabe KN (2006) The Arabidopsis DREB1A gene driven by the stress-inducible rd29A promoter increases salt-stress tolerance in proportion to its copy number in tetrasonic tetraploid potato (Solanum tuberosum). Plant Biotechnol 23:169–177
Benlloch R, Berbel A, Serrano-Mislata A, Madueno F (2007) Floral initiation and inflorescence architecture: a comparative view. Ann Bot 100:659–676
Bihmidine S, Lin J, Stone JM, Awada T, Specht JE, Clemente TE (2012) Activity of the Arabidopsis RD29A and RD29B promoter elements in soybean under water stress. Planta 237:55–64. https://doi.org/10.1007/s00425-012-1740-9
Cervera M, Navarro L, Pena L (2009) Gene stacking in 1-year cycling APETALA1 citrus plants for a rapid evaluation of transgenic traits in reproductive tissues. J Biotechnol 140:278–282
Chica EJ, Albrigo LG (2013) Changes in CsFT transcript abundance at the onset of low-temperature floral induction in sweet orange. J Am Soc Hortic Sci 138:184–189
Davies FS, Albrigo LG (1994) Citrus. CAB International, Wallingford
Duan YX, Fen J, Guo WW (2010) Regeneration and characterization of transgenic kumquat plants containing the Arabidopsis APETALA1 gene. Plant Cell Tissue Organ Cult 100:273–281. https://doi.org/10.1007/s11240-009-9646-3
Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M (2005) Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Res 14:703–712
Estrada-Melo AC, Ma C, Reid MS, Jiang C-Z (2015) Overexpression of an ABA biosynthesis gene using a stress-inducible promoter enhances drought resistance in petunia. Hortic Res. https://doi.org/10.1038/hortres.2015.13
Flachowksy H, Peil A, Sopanen T, Elo A, Hanke V (2007) Overexpression of BpMADS4 from silver birch (Betula pendula Roth.) induces early-flowering in apple (Malus × domestica Borkh.). Plant Breed 126:137–145
Fossi M, Amundson K, Kuppu S, Britt A, Comai L (2019) Regeneration of Solanum tuberosum plants from protoplasts induces widespread genome instability. Plant Physiol 180(1):78–86. https://doi.org/10.1104/pp.18.00906
Goldberg-Moeller R, Shaloma L, Shlizermana L, Samuelsa S, Zura N, Ophira R, Blumwald E, Sadka A (2013) Effects of gibberellin treatment during flowering induction period on global gene expression and the transcription of flowering-control genes in Citrus buds. Plant Sci 198:46–57
Holland N, Saad S, Perl A, Holland D (1996) Tentoxin sensitivity of citrus: cotyledon-dependency of growth inhibition and reversibility of chlorosis. J Exp Bot 47(299):837–842
Ishitani M, Xiong L, Lee H, Stevenson B, Zhu J-K (1998) HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis. Plant Cell 10:1151–1161
Jack T (2004) Molecular and genetic mechanisms of floral control. Plant Cell 16(Suppl 1):S1–S17
Jensen PJ, Makalowska I, Altman N, Fazio G, Praul C, Maximova SN, Crassweller RM, Travis JW, McNellis TW (2010) Rootstock-regulated gene expression patterns in apple tree scions. Tree Genet Genomes 6:57–72
Jupe F, Rivkin AC, Michael TP, Zander M, Motley ST, Sandoval JP, Slotkin RK, Chen H, Castanon R, Nery JR, Ecker JR (2019) The complex architecture and epigenomic impact of plant T-DNA insertions. PLoS Genet 15(1):e1007819. https://doi.org/10.1371/journal.pgen.1007819
Koltunow AM, Soltys K, Nito N, McClure S (1995) Anther, ovule, seed, and nucellar embryo development in Citrus sinensis cv. Valencia. Can J Bot 73:1567–1582
Le Roux P, Flachowsky H, Hanke M, Gessler C, Patocchi A (2012) Use of transgenic early flowering approach in apple (Malus × domestica Borkh.) to introgress fire blight resistance from cultivar Evereste. Mol Breed 30:857–874. https://doi.org/10.1007/s11032-011-9669-4
Li X, Xie R, Lu Z, Zhou Z (2010) The origin of cultivated citrus as inferred from internal transcribed spacer and chloroplast DNA sequence and amplified fragment length polymorphism fingerprints. J Am Soc Hortic Sci 135:341–350
Liu X-Y, Li J, Liu M-M, Yao Q, Chen JZ (2017) Transcriptome profiling to understand the effect of citrus rootstocks on the growth of ‘Shatangju’ mandarin. PLoS ONE 12(1):e0169897. https://doi.org/10.1371/journal.pone.0169897
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Ma H (1994) The unfolding drama of flower development: recent results from genetic and molecular analyses. Genes Dev 8:745–756
Mankin SL, Thompson WF (2001) New green fluorescent protein genes for plant transformation: intron-containing, ER-localized, and soluble-modified. Plant Mol Biol Rep 19:13–26
Msanne J, Lin J, Stone JM, Awada T (2011) Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 234:97–107
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nishikawa F (2013) Review of floral induction in citrus. Jpn Soc Hortic Sci 82(4):283–292
Orbović V, Grosser W (2006) Citrus: sweet orange (Citrus sinensis L. Osbeck ‘Valencia’) and Carrizo citrange [Citrus sinensis (L.) Osbeck × Poncirus trifoliata (L.) Raf.]. In: Wang K (ed) Agrobacterium protocols. Methods in molecular biology. Humana Press Inc., Totowa, pp 177–189
Pena L, Martin-Trillo M, Juarez J, Pina JA, Navarro L, Martinez-Zapater JM (2001) Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nat Biotechnol 19:263–267
Petri C, Alburquerque N, Faize M, Scorza R, Dardick C (2018) Current achievements and future directions in genetic engineering of European plum (Prunus domestica L.). Transgenic Res 27:225–240
Pillitteri LJ, Lovatt CJ, Walling LL (2004a) Isolation and characterization of a TERMINAL FLOWER homolog and its correlation with juvenility in Citrus. Plant Physiol 135:1540–1551
Pillitteri LJ, Lovatt CJ, Walling LL (2004b) Isolation and characterization of LEAFY and APETALA1 homologues from Citrus sinensis L Osbeck ‘Washington.’ J Am Soc Hortic Sci 129:846–856
Prassinos C, Ko JH, Lang G, Iezzoni AF, Han KH (2009) Rootstock-induced dwarfing in cherries is caused by differential cessation of terminal meristem growth and is triggered by rootstock-specific gene regulation. Tree Physiol 29:927–936
Qiu W, Liu M, Qiao G, Jiang J, Xie L, Zhuo R (2012) An isopentyl transferase gene driven by the stress-inducible rd29A promoter improves salinity stress tolerance in transgenic tobacco. Plant Mol Biol Rep 30:519–528
Ritonga FN, Chen S (2020) Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 9(590):1–13. https://doi.org/10.3390/plants9050560
Salonia F, Ciacciuli A, Poles L, Pappalardo HD, La Malfa S, Licciardello C (2020) New plant breeding techniques in citrus for the improvement of important agronomic traits. A review. Front Plant Sci 11:1234. https://doi.org/10.3389/fpls.2020.01234
Samach A (2012) Congratulations, you have been carefully chosen to represent an important developmental regulator! Ann Bot 111:329–333
Shen X, Gmitter FG Jr, Grosser JW (2011) Immature embryo rescue and culture. In: Thorpe T, Yeung E (eds) Plant embryo culture. Methods in molecular biology, vol 710. Humana Press, Totowa, pp 75–92. https://doi.org/10.1007/978-1-61737-988-8_7
Sinn JP, Held J, Vosburg C, Klee SM, Orbovic V, Taylor E, Gottwald T, Stover E, Moore G, McNellis TW (2020) Flowering Locus T chimeric protein induces floral precocity in edible citrus. Plant Biotechnol J. https://doi.org/10.1111/pbi.13463
Stelpflug SC, Eichten SR, Hermanson PJ, Springer NM, Kaeppler SM (2014) Consistent and heritable alterations of DNA methylation are induced by tissue culture in maize. Genetics 198(1):209–218. https://doi.org/10.1534/genetics.114.165480
Stroud H, Ding B, Simon SA, Feng S, Bellizzi M, Pellegrini M, Wang G-L, Meyers BC, Jacobsen SE (2013) Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2:e00354. https://doi.org/10.7554/eLife.00354
Tan F-C, Swain SM (2007) Functional characterization of AP3, SOC1 and WUS homologues from citrus (Citrus sinensis). Physiol Plant 131:481–495
Tang L, Lovatt CJ (2019) Effects of low temperature and gibberellic acid on floral gene expression and floral determinacy in ‘Washington’ navel orange (Citrus sinensis L. Osbeck). Sci Hortic 243:92–100
Weinhold A, Kallenbach M, Baldwin IT (2013) Progressive 35S promoter methylation increases rapidly during vegetative development in transgenic Nicotiana attenuata plants. BMC Plant Biol 13:99. https://doi.org/10.1186/1471-2229-13-99
Xiao H-J, Liu K-K, Li D-W, Arisha MH, Chai W-G, Gong Z-H (2015) Cloning and characterization of the pepper CaPAO gene for defense responses to salt-induced leaf senescence. BMC Biotechnol 15(100):1–12. https://doi.org/10.1186/s12896-015-0213-1
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Zhang J-Z, Mei L, Liu R, Khan MRG, Hu C-G (2014) Possible involvement of locus-specific methylation on expression regulation of LEAFY homologous gene (CiLFY) during precocious trifoliate orange phase change process. PLoS ONE 9(2):e88558. https://doi.org/10.1371/journal.pone.0088558
Acknowledgements
The authors thank Dr. Lynn Pillitteri for the full-length gene sequences for Citrus sinensis APETALA1 and LEAFY and Dr. Martha L. Orozco, Director of the Plant Transformation Research Center, University of California, Riverside, for providing the pCAMBIA2301 plasmid and for helping with the preparation of the transformation vectors containing the CsAP1 and CsLFY genes.
Author information
Authors and Affiliations
Contributions
CJL, VO, and YA designed the experiments, JN and YA constructed binary vectors, VO, SAR, BM, and YA preformed the experiments, all authors wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orbović, V., Ravanfar, S.A., Acanda, Y. et al. Stress-inducible Arabidopsis thaliana RD29A promoter constitutively drives Citrus sinensis APETALA1 and LEAFY expression and precocious flowering in transgenic Citrus spp.. Transgenic Res 30, 687–699 (2021). https://doi.org/10.1007/s11248-021-00260-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-021-00260-z