Abstract
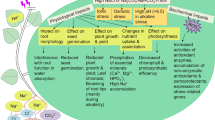
To identify minimal effective promoters for driving abiotic stress-inducible transgene expression in rice, we selected promoter elements of three stress-responsive genes, viz. rab16A coding for dehydrin, OsABA2 coding for zeaxanthin epoxidase, and a gene coding for a hypothetical protein (HP1) based on the presence of ABA-, salt- and drought-responsive cis-acting elements. These were translationally fused to the gusA reporter gene and introduced into rice to study their effect on heterologous gene expression. The OsABA2 promoter was found to be the most effective and desirable promoter among the three in terms of driving a low constitutive transgene expression under normal conditions and high induction in response to ABA, salt and drought stress, the highest being a 12-fold induction in response to ABA. The rab16A and HP1 promoters resulted in high levels of constitutive expression. While induction of GUS activity was generally two- to threefold for all the treatments in roots for both the promoters, induction in leaves was generally insignificant, the exceptions being rab16A in response to continuous salt stress and HP1 in response to water deficit. It was also observed that the three promoters, in general, resulted in lower constitutive expression, but higher induction in roots as compared to leaves.






Similar content being viewed by others
References
Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K (1997) Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid- regulated gene expression. Plant Cell 9:1859–1869
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78. doi:10.1105/tpc.006130
Agrawal GK, Yamazaki M, Kobayashi M, Hirochika R, Miyao A, Hirochika H (2001) Screening of the rice viviparous mutants generated by endogenous retroposons Tos17 insertion. Tagging of a zeaxanthin epoxidase gene and a novel Ostatc gene. Plant Physiol 125(3):1248–1257. doi:10.1104/pp.125.3.1248
Babu RC, Jingxian Z, Blum A, Ho T-HD, Wu R, Nguyen HT (2004) HVA1, a LEA gene from barley confers dehydration tolerance in transgenic rice (Oryza sativa L.) via cell membrane protection. Plant Sci 166:855–862. doi:10.1016/j.plantsci.2003.11.023
Baker SS, Wilhelm KS, Thomashow MF (1994) The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol 24:701–713. doi:10.1007/BF00029852
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58. doi:10.1080/07352680590910410
Bhattacharyya BA, Stermer BA, Dixon RA (1994) Reduced variation in transgene expression from a binary vector with selectable markers at the right and left T-DNA borders. Plant J 6:957–968. doi:10.1046/j.1365-313X.1994.6060957.x
Capell T, Bassie L, Christou P (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101:9909–9914. doi:10.1073/pnas.0306974101
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33:751–763. doi:10.1046/j.1365-313X.2003.01661.x
Garg AK, Kim J-K, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99:15898–15903. doi:10.1073/pnas.252637799
Gorantla M, Babu PR, Lachagari VBR, Reddy AMM, Wusirika R, Bennetzen JL, Reddy AR (2007) Identification of stress responsive genes in an indica rice (Oryza sativa L.) using ESTs generated from drought stressed seedlings. J Exp Bot 58:253–265. doi:10.1093/jxb/erl213
Grace ML, Chandrasekharan MB, Hall TC, Crowe AJ (2004) Sequence and spacing of TATA box elements are critical for accurate initiation from the beta-phaseolin promoter. J Biol Chem 279:8102–8110
Hattori T, Totsuka M, Hobo T, Kagaya Y, Yamamoto-Toyoda A (2002) Experimentally determined sequence requirement of ACGT-containing abscisic acid response element. Plant Cell Physiol 43:136–140. doi:10.1093/pcp/pcf014
Hofgen R, Willmitzer L (1998) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877. doi:10.1093/nar/16.20.9877
Hudson ME, Quail PH (2003) Identification of promoter motifs involved in the network of phytochrome A-regulated gene expression by combined analysis of genomic sequence and microarray data. Plant Physiol 133:1605–1616. doi:10.1104/pp.103.030437
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kaplan B, Davydov O, Knight H, Galon Y, Knight MR, Fluhr R, Fromm H (2006) Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+ -responsive cis elements in Arabidopsis. Plant Cell 18:2733–2748. doi:10.1105/tpc.106.042713
Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch-Martinez N, Krishnan A, Nataraja KN, Udayakumar M, Pereira A (2007) Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci USA 104:5270–15275. doi:10.1073/pnas.0707294104
Karim S, Aronsson H, Ericson H, Pirhonen, Leyman B, Welin B, Mantyla E, Palva ET, Van Dijck P, Holmstrom K-O (2007) Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol Biol 64(4):371–386. doi:10.1007/s11103-007-9159-6
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291. doi:10.1038/7036
Kavikishor PB, Hong Z, Miao G-H, Hu C-AA, Verma DPS (1995) Overexpression of D1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Kim SY, Chung HJ, Thomas TL (1997) Isolation of a novel class of bZIP transcription factors that interact with ABA-responsive and embryo-specification elements in the Dc3 promoter using a modified yeast one-hybrid system. Plant J 11:1237–1251. doi:10.1046/j.1365-313X.1997.11061237.x
Kohli A, Twyman RM, Abranches R, Wegel E, Stoger E, Christou P (2003) Transgene integration, organization and interaction in plants. Plant Mol Biol 52(2):247–258. doi:10.1023/A:1023941407376
Mundy J, Yamaguchi Shinozaki K, Chua N-H (1990) Nuclear proteins bind conserved elements in the abscisic acid responsive promoter of a rice rab gene. Proc Natl Acad Sci USA 87:1406–1410. doi:10.1073/pnas.87.4.1406
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325. doi:10.1093/nar/8.19.4321
Nakashima K, Tran LS, Van Nguyen D, Fujita M, Maruyama K, Todaka D, Ito Y, Hayashi N, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J 51:617–630. doi:10.1111/j.1365-313X.2007.03168.x
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351. doi:10.1104/pp.104.059147
Ono A, Izawa T, Chua N-H, Shimamoto K (1996) The rab16B promoter of rice contains two distinct abscisic acid- response elements. Plant Physiol 112:483–491. doi:10.1104/pp.112.2.483
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Monitoring expression profiles of rice gene under cold, drought, high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel blot analyses. Plant Physiol 133:1755–1767. doi:10.1104/pp.103.025742
Rai M, Datta K, Parkhi V, Tan J, Oliva N, Chawla HS, Datta SK (2007) Variable T-DNA linkage configuration affects inheritance of carotenogenic transgenes and carotenoid accumulation in transgenic indica rice. Plant Cell Rep 26(8):1221–1231. doi:10.1007/s00299-007-0333-8
Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K (2000) Over-expression of a single Ca2+ -dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J 23:319–327. doi:10.1046/j.1365-313x.2000.00787.x
Sieburth LE, Meyerowitz EM (1997) Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell 9:355–365
Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J 33:259–270. doi:10.1046/j.1365-313X.2003.01624.x
Su J, Shen Q, Ho T-HD, Wu R (1998) Dehydration-stress-regulated transgene expression in stably transformed rice plants. Plant Physiol 117:913–922. doi:10.1104/pp.117.3.913
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47(6):969–976
Tran L-SP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16:2481–2498. doi:10.1105/tpc.104.022699
Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97:11632–11637. doi:10.1073/pnas.190309197
Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K (1993) An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. Plant Cell 5:1529–1539
Weise A, Lalonde S, Kuhn C, Frommer WB, Ward JM (2008) Introns control expression of sucrose transporter LeSUT1 in trichomes, companion cells and Iin guard cells. Plant Mol Biol 68(3):251–262. doi:10.1007/s11103-008-9366-9
Xiao B, Huang Y, Tang N, Xiong L (2007) Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor Appl Genet 115(1):35–46
Yamaguchi-Shinozaki K, Shinozaki K (1994) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10(2):88–94. doi:10.1016/j.tplants.2004.12.012
Yamaguchi-Shinozaki K, Masanobu M, Mundy J, Chua N-H (1990) Analysis of an ABA responsive rice gene promoter in transgenic tobacco. Plant Mol Biol 15:905–912. doi:10.1007/BF00039429
Yang T, Poovaiah BW (2002) A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem 277:45049–45058. doi:10.1074/jbc.M207941200
Acknowledgments
The authors are thankful to Drs. Maureen Hanson and Kenneth J. Kemphues for critically reading the manuscript and for their constructive comments. Dr. Wricha Tyagi’s help in preparing the figures is acknowledged. Partial financial support to MR from USAID Agricultural Biotechnology Support Project II is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Wu: Deceased.
This paper is humbly dedicated to the memory of Professor Ray Wu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rai, M., He, C. & Wu, R. Comparative functional analysis of three abiotic stress-inducible promoters in transgenic rice. Transgenic Res 18, 787–799 (2009). https://doi.org/10.1007/s11248-009-9263-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-009-9263-2