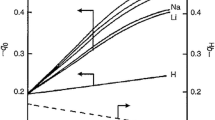
UV–visible spectra of heteropolyacids (HPAs) in solution were measured as a simple method of correlating and predicting the redox properties of HPAs. The range of Keggin-type HPAs included H3PMo12O40, H3PW12O40, and variations of cations, heteroatoms, and/or the framework metals. The absorption edges of 0.01 molar aqueous HPA solutions were determined and compared with absorption edges of solid HPAs and with results from other methods that probe redox properties. The absorption edges of the solutions followed similar trends to the absorption edges of bulk solid HPAs, NDR (negative differential resistance) peak voltages from STM measurements on HPA monolayers, and reduction potentials measured electrochemically. HPAs that are stronger oxidizing agents are characterized by absorption edges at longer wavelength (lower energy), and NDR peaks and reduction potentials at more positive voltages. Comparison of edge energies measured in aqueous solution with the performance of HPA catalysts for liquid phase oxidations demonstrates threshold behavior for oxidation activity in each case examined. These results illustrate the potential use of UV–visible spectroscopy in solution as a simple diagnostic indicator in the selection of HPAs as oxidation catalysts.
Similar content being viewed by others
References
C.L. Hill (ed), Special Thematic Issue on Polyoxometalates. Chem. Rev. 98 (1998) 1
M.S. Kaba I.K. Song D.C. Duncan C.L. Hill M.A. Barteau (1998) Inorg. Chem. 37 398 Occurrence Handle10.1021/ic9705655 Occurrence Handle1:CAS:528:DyaK1cXkvVWlsA%3D%3D
J.B. Moffat (2001) Metal–Oxygen Clusters: The Surface and Catalytic Properties of Heteropoly Oxometalates Kluwer Academic/Plenum Publishers New York
M.T. Pope A. Müller (Eds) (1994) Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity Kluwer Dordrecht
M.T. Pope A. Müller (Eds) (2001) Polyoxometalate Chemistry From Topology via Self-Assembly to Applications Kluwer Dordrecht
J. Melsheimer S.S. Mahmoud G. Mestl R. Schlögl (1999) Catal. Lett. 60 103 Occurrence Handle10.1023/A:1019094621316 Occurrence Handle1:CAS:528:DyaK1MXltFans7k%3D
M.A. Barteau J.E. Lyons I.K. Song (2003) J. Catal. 216 236 Occurrence Handle10.1016/S0021-9517(02)00114-8 Occurrence Handle1:CAS:528:DC%2BD3sXjvVCntbc%3D
J.T. Rhule W.A. Neiwert K.I. Hardcastle B.T. Do C.L. Hill (2001) J. Am. Chem. Soc. 123 12101 Occurrence Handle10.1021/ja015812p Occurrence Handle1:CAS:528:DC%2BD3MXotV2msro%3D
I.A. Weinstock E.M.G. Barbuzzi M.W. Wemple J.J. Cowan R.S. Reiner D.M. Sonnen R.A. Heintz J.S. Bond C.L. Hill (2001) Nature 414 191 Occurrence Handle10.1038/35102545 Occurrence Handle1:CAS:528:DC%2BD3MXosFait7c%3D
J.J. Altenau M.T. Pope R.A. Prados H. So (1975) Inorg. Chem. 14 417 Occurrence Handle10.1021/ic50144a042 Occurrence Handle1:CAS:528:DyaE2MXnvFyiuw%3D%3D
M. Sadakane E. Steckhan (1998) Chem. Rev. 98 219 Occurrence Handle10.1021/cr960403a Occurrence Handle1:CAS:528:DyaK1cXotVSgsQ%3D%3D
M.S. Kaba I.K. Song M.A. Barteau (1997) J. Vac. Sci. Technol. A 15 1299 Occurrence Handle10.1116/1.580580 Occurrence Handle1:CAS:528:DyaK2sXktFSktbY%3D
I.K. Song R.B. Shnitser J.J. Cowan C.L. Hill M.A. Barteau (2002) Inorg. Chem. 41 1292 Occurrence Handle10.1021/ic010832d Occurrence Handle1:CAS:528:DC%2BD38XnvVGltA%3D%3D
I.K. Song M.A. Barteau (2002) J. Mol. Catal. A 182–183 185 Occurrence Handle10.1016/S1381-1169(01)00466-6
K. Chen A.T. Bell E. Iglesia (2002) J. Catal. 209 35 Occurrence Handle10.1006/jcat.2002.3620 Occurrence Handle1:CAS:528:DC%2BD38XkslOktLY%3D
I.K. Song H.S. Kim M.S. Chun (2003) Korean J. Chem. Eng. 20 844 Occurrence Handle1:CAS:528:DC%2BD3sXpsVCgtbk%3D
T. Yamase (1998) Chem. Rev. 98 307 Occurrence Handle10.1021/cr9604043 Occurrence Handle1:CAS:528:DyaK1cXotVSgtg%3D%3D
R.S. Weber (1994) J. Phys. Chem. 98 2999 Occurrence Handle10.1021/j100062a042 Occurrence Handle1:CAS:528:DyaK2cXktVyntLw%3D
J.M. Maestre X. Lopez C. Bo J.-M. Poblet N. Casan-Pastor (2001) J. Am. Chem. Soc. 123 3749 Occurrence Handle10.1021/ja003563j Occurrence Handle1:CAS:528:DC%2BD3MXitFyjtLc%3D
I.K. Song M.A. Barteau (2004) J. Mol. Catal. A 212 229 Occurrence Handle10.1016/j.molcata.2003.10.040 Occurrence Handle1:CAS:528:DC%2BD2cXhtlyitLc%3D
M.H. Youn, H. Him, J.C. Jung, I.K. Song, K.P. Barteau and M.A. Barteau, J. Mol. Catal. A (in press)
G.K. Lee S. Melsheimer S. Berndt G. Mestl R. Schlögl K. Kohler (2001) Appl. Catal. A 214 125 Occurrence Handle10.1016/S0926-860X(01)00485-9 Occurrence Handle1:CAS:528:DC%2BD3MXjtlSru7Y%3D
C.T. Dillon J.H. Holles R.J. Davis J.A. Labinger M.E. Davis (2003) J. Catal. 218 54 Occurrence Handle10.1016/S0021-9517(03)00009-5 Occurrence Handle1:CAS:528:DC%2BD3sXkvVaqsb0%3D
A. Khenkin L. Weiner Y. Wang R. Neumann (2001) J. Am. Chem. Soc. 123 8531 Occurrence Handle10.1021/ja004163z Occurrence Handle1:CAS:528:DC%2BD3MXls12qurY%3D
A. Khenkin Rosenberger R. Neumann (1999) J. Catal. 182 82 Occurrence Handle10.1006/jcat.1998.2323 Occurrence Handle1:CAS:528:DyaK1MXhtF2hs7k%3D
D. Piao K. Inoue H. Shubasaki Y. Taniguchi T. Kitamura Y. Fujiwara (1999) J. Organomet. Chem. 574 116 Occurrence Handle10.1016/S0022-328X(98)00931-0 Occurrence Handle1:CAS:528:DyaK1MXhvVKhsrc%3D
B. El Ali J. Bregeault J. Martin C. Martin (1989) New J. Chem. 13 173 Occurrence Handle1:CAS:528:DyaK3cXhtFygur8%3D
J. Lyons, P. Ellis, H. Myers, G. Suld and W. Langdale, US Patents 4,859,798, 4,803,187 (1989)
B. El Ali A. El-Ghanam M. Tettouhi (2001) J. Mol. Catal. A 165 283 Occurrence Handle10.1016/S1381-1169(00)00453-2 Occurrence Handle1:CAS:528:DC%2BD3MXhtVOitb8%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barteau, K.P., Lyons, J.E., Song, I.K. et al. UV–visible spectroscopy as a probe of heteropolyacid redox properties: application to liquid phase oxidations. Top Catal 41, 55–62 (2006). https://doi.org/10.1007/s11244-006-0094-6
Issue Date:
DOI: https://doi.org/10.1007/s11244-006-0094-6