Abstract
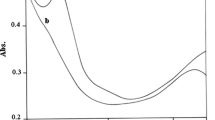
The kinetics of oxidation of 2-mercaptoethanol and 2-mercaptoethylamine by the heteropoly 11-tungsto-1- vanadophosphate anion, [PVVW11O40]4−, have been studied spectrophotometrically in aqueous perchloric acid at 25 °C. EPR and optical studies show that [PVVW11O40]4− is reduced to the one-electron reduced heteropoly blue, [PVIVW11O40]5−, whilst the thiols are oxidized to the corresponding disulphides, RSSR. Spectrophotometric titrations show that the stoichiometry of both reactions is 1:1. At constant pH, the reactions show simple second-order kinetics with first-order dependence of rate on both [oxidant] and [thiol]. At constant [thiol], the rate of the reaction increases with increasing pH. Plots of kobs/[thiol]t versus 1/[H+] are linear with finite intercepts, showing that both the undissociated thiol (RSH) and thiolate ion (RS−) are reactive species. Generation of RS· from RSH proceeds via a separated-concerted proton–electron transfer mechanism. The reaction of thiolate ion is a simple outer-sphere electron transfer reaction. By applying the Marcus theory, the self-exchange rate constants for the couples HOCH2CH2S·/HOCH2CH2S− and H3N+CH2CH2S·/H3N+CH2CH2S− were evaluated as 3 × 109 and 2.2 × 108 M−1 s−1, respectively, at 25 °C.














Similar content being viewed by others
References
Hand CE, Honek JF (2005) J Nat Prod 68:293
Messmore JM, Holmgren SK, Grilley JE, Raines RT (2000) Bioconjugate Chem 11:408
Shena Y, Zhonga L, Markwell S, Cao D (2010) Biochimie 92:530
Jackson MJ (2005) Philos Trans R Soc London Ser B 360:2285
Kemp M, Go YM, Jones DP (2008) Free Radical Biol Med 44:921
Giles GI, Jacop C (2002) Biol Chem 383:375
Singh BK (2005) Asian J Chem 17:1
Adari KK, Nowduri A, Vani P (2006) Transit Met Chem 31:745
Paul PC, Tracey AS (1997) J Biol Inorg Chem 2:644
Wang X, Stanbury DM (2008) Inorg Chem 47:1224
Hung M, Stanbury DM (2005) Inorg Chem 44:3541
Sami P, Venkateshwari K, Mariselvi N, Sarathi A, Rajasekaran K (2009) Transit Met Chem 34:733
Sami P, Venkateshwari K, Mariselvi N, Sarathi A, Rajasekaran K (2010) Transit Met Chem 35:137
Saha B, Hung M, Stanbury DM (2002) Inorg Chem 41:5538
Costentin C, Evans DH, Robert M, Savéant JM, Sing PS (2005) J Am Chem Soc 127:12490
Bonin J, Costentin C, Louault C, Robert M, Routier M, Savéant JM (2010) Proc Natl Acad Sci USA 107:3367
Kapoor RC, Chohan RK, Sinha BP (1971) J Phys Chem 75:2036
Ayoko GA, Olatunji MA (1983) Polyhedron 2:577
Chakraborty M, Mandal PC, Mukhopadhyay S (2012) Polyhedron 45:213
Chakraborty M, Mandal PC, Mukhopadhya S (2013) Inorg Chim Acta 398:77
Chakrabarty S, Banerjee R (2014) Int J Chem Kinetics 47:13
Mishra R, Mukhopadhyay S, Banerjee R (2010) Dalton Trans 39:2692
Sharma VK, Luther GW III, Millero FJ (2011) Chemosphere 82:1083
Vairalakshmi M, Raj V, Sami P, Rajasekaran K (2011) Transit Met Chem 36:875
Sami P, Mariselvi N, Venkateshwari K, Vairalakshmi M, Sarathi A, Rajasekaran K (2010) Transit Met Chem 35:563
Sami P, Anand TD, Premanathan M, Rajasekaran K (2010) Transit Met Chem 35:1019
Smith DP, Pope MT (1993) Inorg Chem 12:331
Smith DP, So H, Bender J, Pope MT (1973) Inorg Chem 12:685
Altenau JJ, Pope MT, Prados RA, So H (1975) Inorg Chem 14:417
Edsall JT, Wyman J (1958) Biophysical chemistry. Academic Press Inc, New York
Warren JJ, Tronic TA, Mayer JM (2011) Chem Rev 110:6951
Ayoko GA, Iyun JF, Ekubo AT (1993) Transit Met Chem 18:6
Bhattarai N, Stanbury DM (2012) Inorg Chem 51:13303
Singh B, Das RS, Banerjee R, Mukhopadhyay S (2014) Inorg Chim Acta 418:51
Surdhar PS, ArmStrong DA (1987) J Phys Chem 91:6532
Mezyk SP, Armstory DA (1999) J Chem Soc Perkin Trans 2:1411
Karmann W, Granzow A, Meissner G, Henglein A (1969) Int J Radiat Phys Chem 1:395
Marcus RA (1963) J Phys Chem 67:853
Marcus RA (1968) J Phys Chem 72:891
Acknowledgments
The authors P.S and K.S thank the University Grants Commission, New Delhi, India, for the award of major research project (F. No. 42-349/2013(SR)) and financial assistance. We also thank Sophisticated Analytical Instrumentation Facility, CECRI Karaikudi, for EPR facilities and Managing Board, Virudhunagar Hindu Nadars’ Senthikumara Nadar College, Virudhunagar for infrastructural facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shanmugaprabha, T., Selvakumar, K., Rajasekaran, K. et al. A kinetic study of the oxidations of 2-mercaptoethanol and 2-mercaptoethylamine by heteropoly 11-tungsto-1- vanadophosphate in aqueous acidic medium. Transition Met Chem 41, 77–85 (2016). https://doi.org/10.1007/s11243-015-9998-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9998-y