Abstract
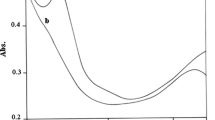
The kinetics of oxidation of phenol and a few ring-substituted phenols by heteropoly 11-tungstophosphovanadate(V), [PVVW11O40]4− (HPA) have been studied spectrophotometrically in aqueous acidic medium containing perchloric acid and also in acetate buffers of several pH values at 25 °C. EPR and optical studies show that HPA is reduced to the one-electron reduced heteropoly blue (HPB) [PVIVW11O40]5−. In acetate buffers, the build up and decay of the intermediate biphenoquinone show the generation of phenoxyl radical (ArO·) in the rate-determining step. At constant pH, the reaction shows simple second-order kinetics with first-order dependence of rate on both [ArOH] and [HPA]. At constant [ArOH], the rate of the reaction increases with increase in pH. The plot of apparent second-order rate constant, k 2, versus 1/[H+] is linear with finite intercept. This shows that both the undissociated phenol (ArOH) and the phenoxide ion (ArO−) are the reactive species. The ArO−–HPA reaction is the dominant pathway in acetate buffer and it proceeds through the OH− ion triggered sequential proton transfer followed by electron transfer (PT-ET) mechanism. The rate constant for ArO−–HPA reaction, calculated using Marcus theory, agrees fairly well with the experimental value. The reactivity of substituted phenoxide ions correlates with the Hammett σ+ constants, and ρ value was found to be −4.8. In acidic medium, ArOH is the reactive species. Retardation of rate for the oxidation of C6H5OD in D2O indicates breaking of the O–H bond in the rate-limiting step. The results of kinetic studies show that the HPA-ArOH reaction proceeds through a concerted proton-coupled electron transfer mechanism in which water acts as proton acceptor (separated-CPET).












Similar content being viewed by others
References
Gunasekar K, Anbarasu K (2009) Croat Chem Acta 82:819
Vanholime R, Morreel K, Ralph J, Boerjan W (2008) Curr Opin Plant Biol 11:278
Huynh MHV, Meyer TJ (2007) Chem Rev 107:5004
Webster RD (2007) Acc Chem Res 40:251
Lansky DE, Goldbreg DP (2006) Inorg Chem 45:5119
Yiu DTY, Lee MFW, Lam WWY, Lau T (2003) Inorg Chem 42:1225
Huang H, Sommerfeld D, Dunn BC, Eyring EH, Lloyd CR (2001) J Phys Chem A 105:3536
Ajlouni AA, Bakac A, Espenson JH (1993) Inorg Chem 32:5792
Nemes A, Bakac A (2001) Inorg Chem 40:746
Bonin J, Costentin C, Louault C, Robert M, Routier M, Saveant JM (2010) Proc Nat Aca Sci USA 107:3367
Song N, Stanbury DM (2008) Inorg Chem 47:11458
Costentin C, Loualt C, Robert M, Saveant JM (2009) Proc Nat Aca Sci USA 106:18143
Galli C, Gentilli P, Pontes ASN, Gamelas JAF, Evtuguin DV (2007) New J Chem 31:1461
Kim YS, Chang H, Kadla JF (2008) J Wood Chem Tec 28:1
Sami P, Rajasekaran K (2009) J Chem Sci 121:155
Sami P, Venkateshwari K, Mariselvi N, Sarathi A, Rajasekaran K (2009) Transit Met Chem 34:733
Sami P, Venkateshwari K, Mariselvi N, Sarathi A, Rajasekaran K (2010) Transit Met Chem 35:137
Sami P, Mariselvi N, Venkateshwari K, Vairalakshmi M, Sarathi A, Rajasekaran K (2010) Transit Met Chem 35:563
Sami P, Mariselvi N, Venkateshwari K, Sarathi A, Rajasekaran K (2010) J Chem Sci 122:335
Sami P, Durai Anand T, Premanathan M, Rajasekaran K (2010) Transit Met Chem 35:1019
Domaille PJ (1984) J Am Chem Soc 106:7677
Smith DP, So H, Bender J, Pope MT (1973) Inorg Chem 12:685
Altenau JJ, Pope MT, Prados RA, So H (1975) Inorg Chem 14:417
Bielski BHJ, Thomas MJ (1987) J Am Chem Soc 109:7761
Hay AS (1969) J Org Chem 34:1160
Rush JD, Cyr JE, Zhao Z, Bielski BH (1995) Free Radical Res 22:349
Grigoriev VA, Hill CL, Weinstock IA (2001): Polyoxometalate oxidation of phenolic lignin models. In: ACS symposium series 785, oxidative delignification chemistry-fundamentals and catalysis, Washington, American Chemical Society, chap. 18, p 297
Um IH, Hong YJ, Kwon DS (1977) Tedrahedron 53:5073
Yuan X, Hualw G, Minsu L (2005) Biomed Environ Sci 18:281
Lee Y, Yoon J, Gunten UV (2005) Environ Sci Technol 39:8978
Marcus RA (1963) J Phy Chem 67:853
Marcus RA (1968) J Phy Chem 72:891
Marcus RA, Eyring H (1964) Annu Rev Phys Chem 15:155
Merenyi G, Lind J, Jhonson M (1993) J Am Chem Soc 115:4945
Schuler RH, Zemel H, Neta P, Fessenden RW (1976) J Am Chem Soc 98:3825
Lind J, Shen X, Eriksen TE, Merenyi G (1990) J Am Chem Soc 112:479
Novoselova IM, Barkovskii VF (1975) Zhur Neorg Khim 20: 1844
Acknowledgments
The authors P. S and K. R thank University Grants Commission, New Delhi, India, for the award of major research project and financial assistance. The authors thank Professor D. M. Stanbury, Auburn University, USA, for helpful discussions during the preparation of the manuscript. We also thank Sophisticated Analytical Instrumentation Facility, Indian Institute of Technology, Bombay, for EPR facilities and Managing Board, Virudhunagar Hindu Nadars’ Senthikumara Nadar College, Virudhunagar, for infrastructural facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vairalakshmi, M., Raj, V., Sami, P. et al. Studies on electron transfer reactions: oxidation of phenol and ring-substituted phenols by heteropoly 11-tungstophosphovanadate(V) in aqueous acidic medium. Transition Met Chem 36, 875–882 (2011). https://doi.org/10.1007/s11243-011-9544-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-011-9544-5