Abstract
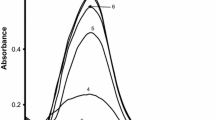
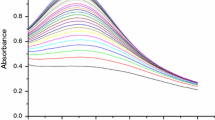
The kinetics and mechanism of the formation of silver nanoparticles by reduction of Ag+ with maltose were studied spectrophotometrically by monitoring the absorbance change at 412 nm in aqueous and micellar media at a temperature range 45–60 °C. The reaction was carried out under pseudo-first-order conditions by taking the [maltose] (>tenfold) the [Ag+]. A mechanism of the reaction between silver ion and maltose is proposed, and the rate equation derived from the mechanism was consistent with the experimental rate law. The effect of surfactants, namely cetyltrimethylammonium bromide (CTAB, a cationic surfactant) and sodium dodecyl sulfate (SDS, an anionic surfactant), on the reaction rate has been studied. The enthalpy and the entropy of the activation were calculated using the transition state theory equation. The particle size of silver sols was characterized by transmission electron microscopy and some physiochemical and spectroscopic tools.









Similar content being viewed by others
References
Anil-Kumar S, Abyanesh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmed M, Khan MI (2007) Biotechnol Lett 29:439
Schultz DA (2003) Curr Opin Biotech 14:13
Gopinath K, Gowri S, Arumugam A (2013) J Nanost Chem 3:68
Nam JM, Thaxton CS, Mirkin CA (2003) Science 301:1884
Haneda M, Kintaichi Y, Inaba M, Hamada H (1998) Catal Today 42:127
Sharma VK, Yngard RA, Lin Y (2009) Adv Colloid Interface Sci 145:83
Sotiriou GA, Pratsinis SE (2010) Environ Sci Technol 44:5649
Wigginton NS, de Titta A, Piccapietra F, Dobias J, Nesatyy VJ, Suter MJF, Bernier-Latmani R (2010) Environ Sci Technol 44:2163
Chen W, Cai W, Zhang L, Wang G (2001) J Colloid Interface Sci 238:291
Frattini A, Pellegri N, Nicastro D, de Sanctis O (2005) Mater Chem Phys 94:148
Patakfalvi R, Viranyl Z, Dekany I (2004) Colloid Polm Sci 283:299
Kiim M, Byun JW, Shin DS, Lee YS (2009) Mat Res Bull 44:334
Al-Thabati SA, Obaid AY, Al-Youbi AO (2009) Colloid Surf B Biointerface 73:284
Khan Z, Al-Thabati SA, Obaid AY, Al-Youbi AO (2010) Colloid Surf B Biointerface 78:143
Sahoo PK, Kalyan Kamal SS, Jagadeesh Kumar T, Sreedhar B, Singh AK, Srivastava SK (2010) Def Sci 59:447
Khan Z, Al-Thabati SA, Obaid AY, Khan ZA, Al-Youbi AO (2012) J Colloid Interface Sci 367:101
Linnert T, Mulvaney P, Henglein A (1990) J Am Chem Soc 112:4657
Henglein A (1998) Chem Mater 10:444
Wiley B, Sun Y, Mayers B (2005) Chem Eur J 11:454
Kapoor S, Lawless D, Kennepohl P, Meisel D, Serpone N (1994) Langmuir 10:3018
Esumi K, Hosoyo T, Yamahira A, Torigoe K (2000) J Colloid Interface Sci 226:346
Sharma VK, Yngard RA, Lin Y (2009) Adv Colloid Interface Sci 145:83
Henglein A (1993) J Phys Chem 97:5457
Heinzman SW, Gamen B (1982) J Am Chem Soc 104:6801
Tan Y, Li Y, Zhu D (2003) J Colloid Interface Sci 258:244
El-Shishtawy RM, Asiri AM, Al-Otabi MM (2011) Spectrochim Acta A 79:1505
Ershov BG, Janata E, Henglein A, Fojtik A (1993) J Phys Chem 97:4589
Wasowicz T, Michalik R (1991) J Phys Chem 37:427
Menger FM, Portony CE (1967) J Am Chem Soc 89:4698
Bunton CA, Savelli G (1986) Adv Phys Org Chem 22:213
Bunton CA (1997) J Mol Liq 72:231
Weaver MJ, Yee EL (1980) Inorg Chem 19:1936
Acknowledgments
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (130-010-D1433). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ewais, H.A. Kinetics and mechanism of the formation of silver nanoparticles by reduction of silver (I) with maltose in the presence of some active surfactants in aqueous medium. Transition Met Chem 39, 487–493 (2014). https://doi.org/10.1007/s11243-014-9823-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9823-z