Abstract
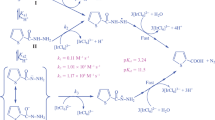
The kinetics of reduction of hexachloroplatinate(IV) by dithionite have been examined spectrophotometrically in sodium acetate–acetic acid buffer medium in the temperature range 288–303 K. The reaction is first order in both platinum(IV) species and dithionite. H+ ion has an inhibiting effect on the rate in the pH range 3.68–4.80. The pseudo-first order rate constant increased upon increasing both ionic strength and dielectric constant. The suggested mechanism involves an initial transition state between two like charged ions, which then decomposes to give SO3 2− through the intermediate formation of free radicals. The presence of free radicals was confirmed by performing the reaction in the presence of acrylamide. PtCl6 2− is finally reduced to PtCl4 2−, as confirmed by thermogravimetric analysis and IR spectrophotometry. The values of ∆H≠ and ∆S≠ associated with the rate-determining step have been calculated as 33 ± 4 kJ mol−1 and −141 ± 7 JK mol−1, respectively. The values of ∆H° and ∆S° for the dissociation of HS2O4 − are 16 ± 4 kJ mol−1 and −14 ± 7 JK mol−1, respectively.






Similar content being viewed by others
References
Mellor JW (1969) A Comprehensive treatise on inorganic and theoretical chemistry, vol 10, Longmans, p 288, 375
Read JF, John J, MacPherson J, Schaubel C, Theriault A (2001) Inorg Chim Acta 315:96–106
Gasana E, Westbroek P, De Wael K, Temmerman E, De Clerck K, Kiekens P (2003) J Electroanalytic Chem 553:35–42
Camacho F, Pfiez MP, Jimhnez MC, Fernmdez M (1997) Chem Eng Science 52:1387–1391
Singh DK, Sharma RN, Srivastava RD (1978) AIChE J 24:232–237
De Wael K, Westbroek P, Temmerman E (2005) Electroanalysis 17:263–268
James J, Hambright P (1973) J Coordination Chem 3:183–185
Cotton FA, Wilkinson G (1988) Advanced inorganic chemistry, 5th edn. Wiley, New York, p 111, 992
Scaife CWJ, Wilkins RG (1980) Inorg Chem 19:3244–3247
Moodley KG, Nicol NJ (1977) J Chem Soc Dalton Trans 3:239–242
Mehrotra US, Agarwal MC, Mushran SP (1970) J Inorg Nucl Chem 32:2325–2329
Sen Gupta KK, Sen PK, Sen Gupta S (1977) Inorg Chem 16:1396–1399
Sen Gupta KK, Sen PK (1977) J Inorg Nucl Chem 39:1651–1653
Sen Gupta KK, Das S, Sen Gupta S (1988) Transition Met Chem 13:155–159
Sen Gupta KK, Maiti S, Sen PK (1981) Transition Met Chem 6:264–266
Sen Gupta KK, Sen PK (1978) J Inorg Nucl Chem 40:1657–1659
Pal B, Sen Gupta KK (2000) Bull Chem Soc Japan 73:553–560
Sen Gupta KK, Begum BA, Ghosh SP (1998) Transition Met Chem 23:295–299
Sen Gupta KK, Begum BA, Pal B (1998) Carbohydr Research 309:303–310
Pal B, Sen Gupta KK, Sen PK (2005) Transition Met Chem 30:593–600
Choi S, Vastag L, Leung C-H, Beard AM, Knowles DE, Larrabee JA (2006) Inorg Chem 45:10108–10114
Lemma K, Berglund J, Farrell N, Elding LI (2000) J Biol Inorg Chem 5:300–306
Hahn FL (1949) Anal Chim Acta 3:62–64
Silverstein RM, Bassler GC, Morril TC (1991) Spectrum identification of organic compounds, 5th edn. Wiley, New York, p 296
McGlynn SP, Kasha M (1956) J Chem Phys 25:481
Rao CNR (1961) Ultraviolet and visible spectroscopy. Butherworks, London, p 29
Kreshkov AP, Yaroslavtsev AA (1977) Course of analytical chemistry, vol 1. Mir Publishers, Moscow, p 345
Frost AA, Pearson RG (1970) Kinetics and mechanism. Wiley Eastern, New Delhi, pp 147, 150
Davies CW (1962) Ion association. Butterworths, London, p 39
Hem WJ, Wayman M (1970) Canad J Chem 48:2278
Lurie JU (1975) Hand book of analytical chemistry. Mir Publication, Moscow, p 300, 310
Latimer WM (1961) The oxidation states of the elements and their potentials in aqueous solution, 2nd edn. Prentice Hall, USA, p 204
Grinberg AA (1962) The chemistry of complex compounds. Pergamon Press, Oxford, p 253
Bailer JC, Emelius HJ, Nyholm DH, Trotman-Dickinson AF (1973) Comprehensive inorganic chemistry, vol 3. Pergamon Press, Oxford, p 189
Taube H (1970) Electron transfer reactions of complex ions in solution. Academic Press, New York
Acknowledgments
Thanks are due to University Grants Commission, New Delhi, India for financial support to B. Pal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pal, B., Sen Gupta, K.K. Kinetics and mechanism of the inner-sphere reduction of hexachloroplatinate(IV) by dithionite in acetate buffer. Transition Met Chem 37, 671–678 (2012). https://doi.org/10.1007/s11243-012-9636-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-012-9636-x