Abstract
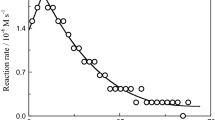
Chromium(III)-lutidinato complexes of general formula [Cr(lutH) n (H2O)6−2n ]3−n (where lutH− is N,O-bonded lutidinic acid anion) were obtained and characterized in solution. Acid-catalysed aquation of [Cr(lutH)3]0 leads to only one ligand dissociation, whereas base hydrolysis produces chromates(III) as a result of subsequent ligand liberation steps. The kinetics of the first ligand dissociation were studied spectrophotometrically, within the 0.1–1.0 M HClO4 and 0.4–1.0 M NaOH range. In acidic media, two reaction stages, the chelate-ring opening and the ligand dissociation, were characterized. The dependencies of pseudo-first-order rate constants on [H+] are as follows: k obs1 = k 1 + k −1/K 1[H+] and k obs2 = k 2 K 2[H+]/(1 + K 2[H+]), where k 1 and k 2 are the rate constants for the chelate-ring opening and the ligand dissociation, respectively, k −1 is the rate constant for the chelate-ring closure, and K 1 and K 2 are the protonation constants of the pyridine nitrogen atom and coordinated 2-carboxylate group in the one-end bonded intermediate, respectively. In alkaline media, the rate constant for the first ligand dissociation depends on [OH−]: k obs1 = k OH(1) + k O[OH−], where k OH(1) and k O are rate constants of the first ligand liberation from the hydroxo- and oxo-forms of the intermediate, respectively, and K 2 is an equilibrium constant between these two protolytic forms. Kinetic parameters were determined and a mechanism for the first ligand dissociation is proposed. The kinetics of the ligand liberation from [Cr(lut)(OH)4]3− were also studied and the values of the pseudo-first-order rate constants are [OH−] independent.












Similar content being viewed by others
References
Vincent JB (2001) Polyherdon 20:1. doi:10.1016/S0277-5387(00)00624-0
Trent LK, Tiedingcancel D (1995) J Sports Med 35:273
Berner TO, Murphy MM, Slesinski RS (2004) Food Chem Toxicol 42:1029. doi:10.1016/j.fct.2004.02.015
Evans GW, Bowman TD (1992) J Inorg Biochem 46:243. doi:10.1016/0162-0134(92)80034-S
Kita E, Gołembiewska K (2007) Transit Met Chem 32:56. doi:10.1007/s11243-006-0128-8
Kita E, Marai H, Zając K (2008) Transit Met Chem 33:211. doi:10.1007/s11243-007-9025-z
Kita E, Marai H, Jasiński M, Drewa T (2008) Transit Met Chem 33:585. doi:10.1007/s11243-008-9084-9
Borowiak-Resterna A, Szymanowski J, Voelkel A (1996) J Radioanal Nucl Chem Art 208:75. doi:10.1007/BF02039750
Agorastos N, Borsig L, Renard A, Antoni P, Viola G, Spingler B, Kurz P, Alberto R (2007) Chem Eur J 13:3824. doi:10.1002/chem.200700031
Shaver A, Hall DA, Ng JB, Lebuis A-M, Hynes RC, Posner BI (1995) Inorg Chim Acta 229:253. doi:10.1016/0020-1693(94)04252-Q
Szorcsik A, Nagy L, Sletten J, Szalontai G, Kamu E, Fiore T, Pellerito L, Kalman E (2004) J Org Chem 689:1145. doi:10.1016/j.jorganchem.2003.11.040
Szorcsik A, Nagy L, Deak A, Scopelliti M, Fekete ZA, Csaszar A, Pellerito C, Pellerito L (2004) J Org Chem 689:2762. doi:10.1016/j.jorganchem.2004.05.045
Szorcsik A, Nagy L, Scopelliti M, Deak A, Pellerito L, Galbacs , Hered M (2006) J Org Chem 691:1622. doi:10.1016/j.jorganchem.2005.12.019
Das A, Pilet G, Luneau D, El Fallah MS, Ribas J, Mitra S (2005) Inorg Chim Acta 358:4581. doi:10.1016/j.ica.2005.07.036
Min D, Yoon SS, Jung D-Y, Lee CY, Kim Y, Han WS, Lee SW (2001) Inorg Chim Acta 324:293. doi:10.1016/S0020-1693(01)00621-1
Zhang X-M (2005) Inorg Chim Acta 358:1865. doi:10.1016/j.ica.2004.12.038
Patrick BO, Stevens CL, Storr A, Thompson RC (2005) Polyhedron 24:2242. doi:10.1016/j.poly.2005.03.085
Mendoza-Diaz G, Rigotti G, Piro OE, Sileo EE (2005) Polyhedron 24:777. doi:10.1016/j.poly.2005.02.007
Liang Y, Hong M, Sun D, Zhao Y, Weng J, Wang R (2002) Inorg Chem Commun 5:366. doi:10.1016/S1387-7003(02)00385-4
Min D, Lee SW (2002) Inorg Chem Commun 5:978. doi:10.1016/S1387-7003(02)00630-5
Huang Y-G, Zhou Y-F, Yuan D-Q, Wu B-L, Jiang F-L, Hong M-C (2007) J Mol Struct 830:85. doi:10.1016/j.molstruc.2006.07.001
Sileo EE, de Araujo AS, Rigotti G, Piro OE, Castellano EE (2003) J Mol Struct 644:67. doi:10.1016/S0022-2860(02)00450-7
Pan L, Frydek T, Sander MB, Huang X, Li J (2001) Inorg Chem 40:1271. doi:10.1021/ic001012o
Rao L, Zhang Z, Friese JI, Ritherdon B, Clarck SB, Hess NJ, Rai D (2002) J Chem Soc Dalton Trans 267 doi:10.1039/b104154c
Richens DT (2005) Chem Rev 105:1961
Kita E, Szabłowicz M (2003) Transit Met Chem 28:698. doi:10.1023/A:1025469431212
Szabłowicz M, Kita E (2004) Transit Met Chem 29:762. doi:10.1007/s11243-004-9115-0
Acknowledgements
(i) The authors wish to thank to Authorities of N. Copernicus University for the financial support of these studies with the Grant No. Ch-368; (ii) Hasan Marai wishes to thank to Libyan Government for financial support of his Ph.D. studies in Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kita, E., Marai, H. & Iglewski, Ł. Chromium(III) complexes with lutidinic acid: kinetic studies in HClO4 and NaOH solutions. Transition Met Chem 34, 75–84 (2009). https://doi.org/10.1007/s11243-008-9160-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-008-9160-1