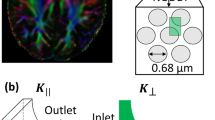
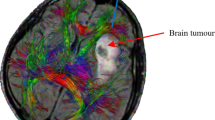
Abstract
Convection-enhanced drug delivery is a technique where a therapeutic agent is infused under positive pressure directly into the brain tissue. For predicting the final concentration distribution and optimizing infusion rate and catheter placement, numerical models can be of great help. However, despite advances in modeling this process, often the infused agent does not reach the targeted region prescribed in the modeling phase. In this study, patient-specific brain structure and parameters, obtained from diffusion tensor imaging (DTI), are implemented in a numerical model which describes the flow and transport in an elastic deformable matrix. To our knowledge, this is the first time that information from DTI is used in a numerical model which includes both transport of a therapeutic agent and tissue deformation. Fractional anisotropy (FA) is used to distinguish between gray and white matter and tortuosity to differentiate between inside and outside the brain tissue. One voxel in the DT-image is represented by one element of the numerical grid. The DT-images were in addition used to determine the orientation of the white matter fiber tracts and calibrate permeability and diffusion coefficients found in the literature. Values chosen for the porosity and Lamé parameters are also based on those found in the literature. Given realistic literature values, the calibration of the permeability and diffusion tensors are shown to be successful. Our result shows that preferential flow occur in direction of the white matter fiber tracts. The current model assumes linear deformation, corresponding to small porosity changes. But, because large porosity changes occur that may adversely affect drug transport, non-linear deformations should be included in the future.
Similar content being viewed by others
References
Baish J.W., Netti P.A., Jain R.K.: Transmural coupling of fluid flow in microcirculatory network and interstitium in tumors. Microvasc. Res. 53, 128–141 (1997)
Basser P.: Interstitial pressure, volume, and flow during infusion into brain tissue. Microvas. Res. 44, 143–165 (1992)
Basser P., Pierpaoli C.: Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor mri. J. Magn. Reson. B 111, 209–219 (1996)
Basser P., Mattiello J., Lebihan D.: Mr diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267 (1994a)
Basser P., Mattielo J., Lebihan D.: Estimation of the effective self-diffusion tensor from the nmr spin-echo. J. Magn. Reson. B 103, 247–254 (1994b)
Baxter L., Jain R.: Transport of fluid and macromolecules in tumors; the role of interstitial pressure and convection. Microvasc. Res. 37, 77–104 (1989)
Bender B., Klose U.: Cerebrospinal fluid and interstitial fluid volume measurements in the human brain at 3t with epi. Magn. Reson. Med. 61, 834–841 (2009)
Biot M.: Theory of elasticity and consolidation for a porous anisotropic solid. J. Appl. Phys. 25, 182–185 (1955)
Bobo R., Akbasak D.W.A.L., Morrison P., Dedrick R., Oldfield E.: Convection-enhanced delivery of macromolecules in the brain. Proc. Natl Acad. Sci. USA 91, 2076–2080 (1994)
Brown W.: Solid mixture permittivities. J. Chem. Phys. 23, 1514–1517 (1955)
Chen X., Sarntinoranont M.: Biphasic finite element model of solute transport for direct infusion into nervous tissue. Ann. Biomed. Eng. 35, 2145–2158 (2007)
Chen Z., Broaddus W., Viswanathan R., Raghavan R., Gillies G.: Intraparenchymal drug delivery via positive-pressure infusion: Experimental and modeling studies of poroelasticity in brain phantom gels. IEEE Trans. Biomed. Eng. 49(2), 85–96 (2002)
Cheng S., Bilston L.E.: Unconfined compression of white matter. J. Biomech. 40, 117–124 (2007)
Cheng S., Clarke E., Bilston L.: Rheological properties of the tissues of the central nervous system: a review. Med. Eng. Phys. 30, 1318–1337 (2008)
Cowin S., Cardoso L.: Fabric dependence of wave propagation in anisotropic porous media. Biomech. Model. Mechanobiol. 10, 39–65 (2011)
Dutta-Roy T., Wittek A., Miller K.: Biomechanical modelling of normal pressure hydrocephalus. J. Biomech. 41, 2263–2271 (2008)
Flemisch, B., Darcis, M., Erbertseder, K., Faigle, B., Lauser, A., Mosthaf, K., Muthing, S., Nuske, P., Tatomir, A., Wolff, M., Helmig, R.: DuMux: DUNE for multi-{phase, component, scale, physics, ...} flow and transport in porous media. Adv. Water Resour. Corrected Proofs, doi:10.1016/j.advwatres.2011.03.007 (2011)
Garcia J., Smith J.: A biphasic hyperelastic model for the analysis of fluid and mass transport in brain tissue. Ann. Biomed. Eng. 37, 375–386 (2009)
Gillies G., Smith J., Humphrey J., Broaddus W.: Positive pressure infusion of therapeutic agents into brain tissues: Mathematical and experimental simulations. Technol. Health Care 13, 235–243 (2005)
Groothuis R.: The blood-brain and blood-tumor barriers: a review of stragies for increasing drug delivery. Neuro-Oncology 2, 45–59 (2000)
Hagmann P., Jonasson L., Maeder P., Thiran J., Wedeen V., Meuli R.: Understanding diffusion mr imaging techniques: From scalar diffusion weighted imagin to diffusion tensor imaging and beyond. RadioGraphics 26, S205–S223 (2006)
Hassanizadeh M., Gray W.: General conservation equations for multi phase systems: 3. constitutive theory for porous media flow. Adv. Water Resour. 3, 25–40 (1980)
Helmig R.: Multiphase Flow and Transport Processes in the Subsurface. Springer, Heidelberg (1997)
Holz M., Hei S., Sacco A.: Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1h nmr pfg measurements. Phys. Chem. Chem. Phys. 2, 4740–4742 (2000)
Kaczmarek M., Subramaniam R., Neff S.: The hydromechanics of hydrocephalus: steady-state solutions for cylindrical geometry. Bull. Math. Biol. 59(2), 295–323 (1997)
Kalyanasundaram S., Calhoun V., Leong K.: A finite element model for predicting the distribution of drugs intracranially to the brain. Am. J. Physiol. 273, R1810–R1821 (1997)
Kim H., Lizak M., Tansey G., Csaky K., Robinson M., Yuan P., Wang N., Lutz R.: Study of ocular transport of drugs released from an intravitreal implant using magnetic resonance imaging. Ann. Biomed. Eng. 33(2), 150–164 (2005)
Kim J., Garett G., Chen X., Mareci T., Sarntinoranont M.: Voxelized model of interstitial transport in the rat spinal cord following direct infusion into white matter. J. Biomech. Eng. 131, 071,007 (2009)
Kim J.H., Mareci T., Sarntinoranont M.: A voxelized model of direct infusion into the corpus callosum and hippocampus of the rat brain: model development and parameter analysis. Med. Biol. Eng. Comput. 48, 203–214 (2010)
Klatt D., Hamhaber U., Asbach P., Braun J., Sack I.: Noninvasive assessment of the rheological behavior of human organs using multifrequency mr elastography: a study of brain and liver viscoelasticity. Phys. Med. Biol. 52, 7281–7294 (2007)
Lai W., Mow W.: Drag-induced compression of articular cartilage during a permeation experiment. Biorheology 17(1–2), 111–123 (1980)
Linninger A., Somayaji M., Erickson T., Guo X., Penn R.: Computational methods for predicting drug transport. J. Biomech. 41, 2176–2178 (2008a)
Linninger A., Somayaji M., Mekarsk M., Zhang L.: Prediction of convection-enhanced drug delivery to the human brain. J. Theor. Biol. 250, 125–138 (2008b)
McGuire S., Zaharoff D., Yuan F.: Nonlinear dependence of hydraulic conductivity on tissue deformation during intratumoral infusion. AnnBiomedEng 37(7), 1173–1181 (2006)
Miller K., Chinzei K.: Mechanical properties of brain tissue in tension. J. Biomech. 35, 483–490 (2002)
Morrison P.F., Laske D.W., Bobo H., Oldfield E., Dedrick R.: High-flow microinfusion: tissue penetration and pharmacodynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 266, 292–305 (1994)
Netti P., Baxter L., Boucher Y., Skalak R., Rakesh K.: Time-dependent behavior of interstitial fluid pressure in solid tumors: implications for drug delivery. Cancer Res. 55, 5451–5458 (1995)
Netti P., Baxter L., Boucher Y., Skalak R., Jain R.: Macro- and microscopic fluid transport in living tissues: applications to solid tumors. AIChE J. 43(3), 818–831 (1997)
Nicholson C.: Diffusion and related transport mechanisms in the brain tissue. Rep. Progr. Phys. 64, 815–884 (2001)
Odgaard A.: Three-dimensional methods for quantification of cancellous bone architecture. Bone 20(4), 315–328 (1997)
Odgaard A., Kabel J., van Rietbergen B., Dalstra M., Huiskes R.: Fabric and elastic principal directions of cancellous bone are closely related. J. Biomech. 30(5), 487–495 (1997)
Prabhu S., Broaddus W., Gillies G., Loudon W., Chen Z.J., Smith B.: Distribution of macromolecular dyes in brain using positivepressure infusion: a model for direct controlled delivery of therapeutic agents. Surg. Neurol. 50, 367–375 (1998)
Raghavan R., Brady M., Rodriguez-Ponze M., Hartlep A., Pedain C., Sampson J.: Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg. Focus 20(3), E12 (2006)
Sarntinoranont M., Banerjee R., Lonser R., Morrison P.: A computational model of direct interstitial infusion of macromolecules into spinal cord. Ann. Biomed. Eng. 31(4), 448–461 (2003)
Sarntinoranont M., Chen X., Zhao J., Mareci T.: Computational model of interstitial transport in the spinal cord using diffusion tensor imaging. Ann. Biomed. Eng. 34, 1304–1321 (2006)
Sen A., Torquato S.: Effective conductivity of anisotropic two-phase composite media. Phys. Rev. B 39, 4504–4515 (1988)
Smith J., Humphrey A.: Interstitial transport and transvascular fluid exchange during infusion into brain and tumor tissue. Microvasc. Res. 73, 58–73 (2007)
Smith J.A., Garcia J.A.: A nonlinear biphasic model of flow-controlled infusion in brain: fluid transport and tissue deformation analyses. J. Biomech. 42, 2017–2025 (2009)
Smith J., Garcia J.: A nonlinear biphasic model of flow-controlled infusions in brain: mass transport analyses. J. Biomech. 44, 524–531 (2011)
Taylor Z., Miller K.: Reassessment of brain elasticity for analyses of biomechanisms of hydocephalus. J. Biomech. 37, 1263–1269 (2004)
Tuch D., Wedeen V., Dale A., George J., Belliveau J.: Conductivity tensor mapping of the human brain using diffusion tensor mri. Proc. Natl Acad. Sci. 98, 11697–11701 (2001)
Vorisek I., Sykova E.: Measuring diffusion parameters in the brain: comparing the real-time iontophoretic method and diffusion-weighted magnetic resonance. Acta Physiol. 195, 101–110 (2009)
Zhang X.Y., Luck J., Dewhirst W., Yuan F.: Interstitial hydraulic conductivity in a fibrosarcoma. Am. J. Physiol. 279, H2726–H2734 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Støverud, K.H., Darcis, M., Helmig, R. et al. Modeling Concentration Distribution and Deformation During Convection-Enhanced Drug Delivery into Brain Tissue. Transp Porous Med 92, 119–143 (2012). https://doi.org/10.1007/s11242-011-9894-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-011-9894-7