Abstract
Somatic embryogenesis, as a promising biotechnological tool for many conifer trees, has never been applied for the Abies nebrodensis species. Although all the encouraging results previously obtained by the EU LIFE (European LIFE program) funded projects in over ten years, the critically endangered Sicilian fir remains alarmingly close to extinction. In this study, we reported the first protocol of somatic embryogenesis obtained from mature zygotic embryos of the Abies nebrodensis. Seeds from Abies adult trees with specific identification numbers (IN) were collected and full seeds were identified by X-ray. Different experiments were carried out for callus initiation, from both zygotic immature and mature embryos, testing different culture media. The immature embryos did not give embryogenic tissue (ET). Embryogenic callus (EC) was successfully induced from mature embryos with variable frequencies (0–40%). Schenk and Hilderbrandt (SH) was the most suitable initiation medium where the obtained callus initiation rate reached up to 40% for IN7 (first experiment). 6-benzylaminopurine (BAP) showed to be essential to induce EC (second experiment). IN8 presented the highest callus initiation rate (40%) among all tested donor trees, whereas IN13 recorded the lowest rate with 4% (third experiment). ET maturation from each singular embryo of IN7, IN8, IN10 and IN21 was successfully achieved in SH medium containing 37,83 µM abscisic acid (ABA), 8% of polyethylene glycol (PEG-4000) and 4% maltose. The encapsulation technology was assessed on the obtained ET and its proliferation was observed after encapsulation.
Key message
A protocol for somatic embryogenesis from mature embryos of the Abies nebrodensis was achieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sicilian fir (Abies nebrodensis Mattei) is the rarest endemic coniferous tree, in the Madonie Regional Natural Park, mountainous area of Polizzi Generosa, in north-central Sicily, Italy (Pasta et al. 2020). In ancient times, the wood of this species was extensively used because of its elasticity and resistance, making it valued in private and religious constructions, such as doors and roof-beams of local churches. Its popularity contributed to the danger of extinction by 1900, but it was rediscovered in 1957 a few kilometers from Polizzi Generosa, in a small area (Silva 2008). Many factors made the propagation of this species difficult, such as the scarcity of embryos in seeds and their low germination rate, added to other dangers, like hybridization with non-native firs, leading to massive genetic pollution (Scialabba et al. 2009; Thomas 2017). The strong genetic erosion and low natural regeneration have limited the residual population to only thirty currently existing mature trees, leading to its classification as a critically endangered species by the International Union for Conservation of Nature (IUCN). According to LIFE nature projects evaluation, interventions for the conservation of the Sicilian fir were started from the ‘40 s of the last century. Despite the important and successful efforts previously carried out to preserve in situ and ex situ Abies nebrodensis Mattei—(EU LIFE four-year ‘LIFE-Natura 2000’ funded project 2001–2005), Sicilian fir needs to be further safeguarded by innovative methods. Therefore, the current LIFE4FIR project for ‘Decisive in situ and ex situ conservation strategies to secure the critically endangered Sicilian fir, Abies nebrodensis—LIFE 18 NAT/IT/164 LIFE4FIR’, was funded (EU LIFE four-year funded project 2019–2023), with the aim to save and support this precious species.
Somatic embryogenesis is considered as an advanced tool applied for the forestry trees and was first reported since more than thirty years for coniferous species. The first application for Norway spruce proved to be a promising micropropagation approach (Hakman et al. 1985), then this became a beneficial method for ecologically and economically important forestry species. For Norway spruce (Picea abies L. Karst.) and Scots pine (Pinus sylvestris L.) further research has been established to evaluate the mechanisms controlling tree embryogenesis and in vitro cultivation (Hazubska-Przybył et al. 2022).
For the genus Abies, this approach was first described by Schuller et al. (1989) and this process has been applied in diverse fir species (Abies alba × Abies nordmanniana; Abies alba × Abies cephalonica) (Vookovà et al. 1997; Salajová et al. 1996; Salajová and Salaj 2003). This technique in combination with cryoconservation of the embryogenic tissue allows a large-scale propagation and preservation of forestry resources (Lelu-Walter et al. 2013). Emblings have been produced on a large scale from somatic embryos of commercially important species, and large field trials have been established (Loyola and Ochoa 2016, Isah 2016). For Abies nebrodenisis, embryogenic callus has never been obtained (Krajňáková et al. 2014).
The main objective of the present paper is to develop highly reproducible in vitro approaches, particularly somatic embryogenesis and encapsulation technology.
Materials and methods
Plant material: donor trees and seeds collection
Individuals selected in the present study are the critically endangered adult trees of Abies nebrodensis, endemic to the north-central part of Sicily. The population is limited to thirty residual individuals, distributed discontinuously in around 100 hectares in the Madonie park, in the ‘Vallone Madonna degli Angeli’ (approximate location 37,85°N, 14,04°E), Polizzi Generosa (Sicily). Cones were collected and stored dry at 4 °C before culture initiation. Immature and mature cones were harvested, after open-pollination. Immature cones containing seeds at late embryogeny stage, were collected at two different dates: mid- and late-July 2020, while mature cones were harvested during the last week of September 2020.
Sterilization
Cones were washed with detergent for about 30 min, then flushed under running water for 4 h (h). Under laminar flow hood, seed scales were removed and treated with 70% ethanol on a clean bench for 1 min (min) for immature seeds and 5 min for mature seeds, then rinsed five times with sterile distilled water. Seeds were then immersed in 20% (v/v) sodium hypochlorite solution to which was added a few drops of Tween 20 for 20 min and rinsed 3 times with sterile water. Later, the seed coat was removed from the immature seeds and the megagametophytes were used as explants. After sterilization, mature seeds were imbibed in sterilized distilled water for 48 h under antiseptic conditions then, zygotic embryos were carefully excised from seeds using sterile forceps.
Selection of full seeds
Two replicates of 50 seeds from each tree were radiographed, with the aid of digital equipment (Gilardoni radio light, Lecco, Italy) connected to a computer. The X-ray plates were evaluated based on both embryo presence and morphology. Seeds previously subjected to the X-Ray imaging test were checked on X-Ray Viewer Screen. Based on the X-ray image, seeds were classified into normal (with embryos, Fig. 1a) and empty (no embryos, Fig. 1b). Empty seeds were separated from the full ones and verified by opening the seed sample under the stereoscope. The percentage of seeds with or without embryo was determined. Furthermore, the maintenance of full-seed viability after the X-ray was evaluated through embryo germination test into hormone free Schenk and Hilderbrandt (1972) basal salt medium (SH).
Callus induction
Callus induction from immature embryos
Megagametophytes were aseptically excised from immature seeds and placed onto various culture media, supplemented with different concentrations of plant growth regulators (PGRs) such as 6-benzylaminopurine (BAP) and 2,4-dichlorophenoxyacetic acid (2,4-D) and testing different carbon sources (either maltose, sucrose, galactose, or lactose) (Table 1). Proliferation of obtained calli was tested on the same medium used for initiation (IM8), as well as on three other different media: (1) Driver Kuniyuki Walnut by Driver and Kuniyuki 1984 (DKW) without PGRs, (2) DWK without plant growth regulators but containing 0.1% active charcoal (AC), and (3) DWK with 4,43 µM BAP, 500 mg L−1 glutamine, and 1 g L−1 casein hydrolysate. All cultures were maintained in the dark at 24 ± 1 °C for 8 weeks.
Callus induction and proliferation from mature embryos
The research activity was performed through three experiments since only a limited number of seeds was available. In the first experiment mature seeds, previously conserved at 4 °C for 6 months, were cultured on both Murashige and Skoog 1962 (MS) and SH media supplemented or not with 4,43 µM of BAP (Table 2). A total of 440 mature embryos from the following donor trees with identification number (IN): IN6, IN7, IN8, IN10, IN12, IN13, IN21, IN22 and IN29 were excised and cultured horizontally on different culture media for callus induction. Then, depending on the highest callus induction rates, the suitable medium was selected. Only embryos from the following donor trees IN7, IN8, IN10, IN13 and IN21 were subjected to statistical analysis according to the availability of embryos with homogenous number.
Once the medium was chosen, a second experiment was established in which zygotic embryos were excised from seeds of the following donor trees IN8, IN10, IN13 and IN21, previously conserved at 4 °C, for more than one year and cultured with 4,43 µM of BAP and/or 4,52 µM of 2,4-D (Table 3). The effect of the donor tree was not studied in this experiment.
Later, in a third experiment, the donor tree effect on somatic embryogenesis induction was evaluated, using a fixed number of 60 mature zygotic embryos from the same donor trees of the second experiment cultured on SH media supplemented with 4,43 µM of BAP and kept in the dark at 24 ± 1 °C. Two weeks after the initiation, embryogenic callus was isolated carefully from the embryo and transferred onto a fresh medium. Embryogenic tissue (ET) was proliferated and sub-cultured onto a fresh medium every 4 weeks.
Somatic embryo development, maturation, and germination
Cell lines (ET derived from each individual mature embryo) were transferred onto SH basal salt medium, supplemented with 4,27 µM abscisic acid (ABA), 8% polyethylene glycol (PEG-4000) and 4% of maltose (Salajová 2003). Cultures were transferred to a fresh medium every 4 weeks and kept at 24 ± 1 °C in darkness. ET formation was continuously observed under the microscope, along with the development of somatic embryos.
Only somatic embryos with a full cotyledonary shape were used. They were isolated from callus and placed carefully on the top surface of a dry and sterile filter paper for three hours for a partial desiccation. Subsequently, two treatments were evaluated, half strength SH basal medium (G1) and full-strength MS medium (G2), both equally enriched with 1% of both sucrose and AC, using or not a disc of filter paper placed on the top of the medium. Ten embryos per germination treatment were placed horizontally in Petri dishes containing 20 mL of basal medium. Plates were incubated at 4 °C for 21 days in the dark and then maintained at 24 ± 1 °C under light conditions (16/8 h light/dark photoperiod).
Encapsulation and cryopreservation assay
Cryopreservation is a useful method for long-term preservation of plant germplasm. Small amount (0,5 g) of embryogenic callus from IN8 and IN10 were encapsulated in alginate beads according to Micheli and Standardi protocol (2016). Later, they were washed with sterilized distilled water, then submerged in 3% sodium alginate solution (w/v). Drops of encapsulating matrix containing ET were then transferred for 20 min into the MS basal medium supplemented with 11,1 mg L−1calcium chloride (CaCl2·2H2O) to obtain ET beads. After washing in sterile water, for cryopreservation protocol, the beads were treated with loading solution (Matsumoto et al. 1994), transferred to liquid solution containing MS medium supplemented with 34,2% of sucrose. Subsequently, a part of ET beads was treated with Plant Vitrification Solution 2 (PVS2) at 0 °C (Sakai et al. 1990), testing different times of contact with the solution (60, 90 and 120 min) and another part of ET beads were used as control. All the ET beads were immersed in liquid nitrogen. After thawing in a water bath (2 min at 40 °C), the beads with ET were transferred onto SH medium supplemented with 4,43 µM of BAP (same medium used for proliferation) to test the viability through the ET proliferation.
Data analysis
The callus induction rate from mature zygotic embryos was evaluated as follows:
Recorded data were statistically analyzed using R software (R version 4.2.1 issued by the R Foundation for Statistical Computing, 2022). Data given in percentages were subjected to arcsine transformation before statistical analysis, significance was determined by analysis of variance (ANOVA) and the difference between the means was compared by LSD test. In the maturation phase, four cell lines (Ab1, Ab2, Ab3 and Ab4) were selected to count the number of pre-cotyledonary and cotyledonary somatic embryos produced, and no statistical analysis was conducted due to the limited number of cell lines.
Results
Selection of seeds
The results showed 100% of survival through an in vitro germination test for embryos issued from tree IN10, i.e., the same germination rate as for seeds not subjected to X-ray, thereby confirming that X-ray inspection is a non-destructive method.
The percentage of full seeds varied among all tested A. nebrodensis adult trees with a mean of 27,4% (Table 4). The X-ray radiography test revealed that seeds from trees with IN7, IN8, IN10 and IN21 presented the largest number of full seeds (Fig. 1a). IN7 has the highest percentage of full seeds with 54%, while IN19 showed 100% of empty seeds (Fig. 1b).
Callus induction
Callus induction from immature zygotic embryos
The addition of the cytokinin (BAP) to the induction medium has shown a positive response on the non-embryogenic callus (NEC) initiation, either alone or in combination with 2,4-D. On media IM6, IM7, IM9 and IM11 (containing BAP), megagametophytes from IN10, IN8 and IN7 showed a percentage of up to 100% of NEC initiation. The formation of watery tissue in the micropylar end of the embryo and a swelling in the middle of the megagametophyte were observed. After one to three months from culture initiation, compact and NEC with unorganized and dedifferentiated cell masses were obtained from immature embryos of three explants on IM11. After induction, the tissue callus proliferated only in the presence of AC but then, after subsequent transfers, proliferation was arrested.
Callus induction from mature zygotic embryos
Experiment 1: Selection of basalt salt medium
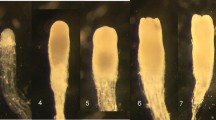
Mature zygotic embryos were able to induce the embryogenic callus (EC) only in the presence of cytokinin (BAP) using the medium T3 (Table 2). Pale green cotyledons and slightly swollen hypocotyls proliferated (Fig. 2a), after two weeks from culture initiation in IN7 embryos, while NEC started to appear in the radicular area. After ten weeks, transparent white ET were observed from the hypocotyl for the first time, according to our knowledge, for this species on T3 medium (Fig. 2b and c). T3 medium showed to be the best initiation medium for callus induction if compared to T1, T2 and T4. A low overall embryogenic callus initiation rate was obtained from all tested embryos (14%), highly related to both donor trees and media used. The highest callus induction rate was registered in this first experiment, for embryos from tree IN7 with 40%, followed by IN21 (15%), IN13 (10%) and IN10 (5%). In this experiment embryos from IN8 did not respond in any tested media (Table 5).
Microscopic observation of morphological heterogeneity of calli induced from mature zygotic embryos of Abies nebrodensis. a Zygotic embryo after two weeks in initiation medium showed swelled hypocotyl and green cotyledons b ET tissue initiated from mature embryo from hypocotyl area c ET tissue initiated from mature embryo with apparition of globular green callus and NEC necrotised. d Watery non-embryogenic callus turning brown. e Watery callus appeared in the cotyledons on medium supplemented with auxin. f; g Watery callus appeared on different parts of zygotic embryo on medium supplemented with auxin. h. Compact callus necrotised after period in culture. i Green globular callus. Bars (a, b, c, d, e, f, g, h) = 1 mm
Experiment 2: Effect of PGR on somatic embryogenesis
Since SH basal salt medium (T3) revealed the highest callus induction rate with up to 40% (Table 5), it was selected to test the best PGRs, as a second experiment. After 10 weeks from culture initiation, the highest embryogenic callus induction rate (5%) was registered for medium supplemented only with 4,43 µM BAP, followed by M3 medium (3,5%) supplemented with 4,43 µM of BAP and 4,52 µM of 2,4-D. M2 medium, enriched only with 4, 52 µM of 2,4-D, that enhanced the development of the NEC to 100% after two weeks from the cotyledons, hypocotyl, and radicular area of the zygotic embryo (Table 3; Fig. 2d, e, f and g). The obtained callus presented a soft and watery aspect, without any further proliferation and finished to be necrotised (Fig. 2h). PGRs used showed a significant effect on the type of callus obtained. NEC showed a high induction rate (76,7–80,1%), in all media supplemented with PGRs, while no initiation occurred in the hormone free medium M0. EC initiation was highly dependent on the presence of BAP, with an induction rate ranging between 3,5 and 4,6% for M3 and M1, respectively. Obtained results regarding the induction rates could be affected by the storage period of cones. Statistical analysis revealed no significant difference between PGRs used in M3 (BAP combined with 2,4-D) if compared to M1 (only BAP) and M2 (only 2,4-D). While M1 was statistically different from M2 revealing the importance of BAP on the induction of the EC rates (Table 6).
Experiment 3: Effect of donor tree on somatic embryogenesis
Results showed that the donor tree has a significant effect on ET initiation from Abies nebrodensis. Callus induction rate through time was evaluated (Fig. 3). The first EC proliferation was noted after two weeks from culture initiation for trees IN10 and IN21, while embryos from trees IN8 and IN13 started EC proliferation from the fourth week. The highest EC induction rate was reached by embryos isolated from IN8 (40%) at the 12th week, which showed to be stable until the 16th week. Embryos from IN10 and IN21 donor trees reached their maximal and uniform EC induction rate from the 14th week until the 16th week levels. Embryos from the IN10 revealed a high EC induction rate (25%) followed by IN21 (15%) and IN13 (4%). The latter tree reached its maximal and stable EC induction rate at the 10th week.
Somatic embryo development, maturation, and germination
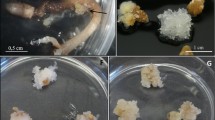
The ET proliferation initiated exclusively from hypocotyls (Fig. 2b and c), while non-ET proliferated from the different parts of zygotic embryo on media supplemented with auxin and/or cytokinin (Fig. 2d, e, f and g). The formed tissue was soft, watery, or even compact and ultimately showed no further proliferation and a necrotised aspect (Fig. 2h). On the other hand, green globular callus was observed on medium supplemented with only BAP (Fig. 2i). This type of callus can be further investigated for organogenesis experiments. The obtained embryogenic tissue was white, transparent, alongside mucilaginous masses with the formation of the embryonal-suspensor masses (Fig. 4a). Only EC was able to proliferate and to differentiate into cell lines able to produce pre-cotyledonary somatic embryos. Cell lines were maintained on a fresh proliferation medium by subcultures every 2 to 3 weeks of intervals. All proliferated ET were transferred onto the maturation medium. In total from all the experiments, 25 embryogenic cell lines were maintained and persisted until the maturation stage. After three to five weeks, pre-cotyledonary somatic embryos became visible as structures attached to the ET by suspensors and a clear embryonic region (Fig. 4b). Later in the same medium, somatic embryos with a clear cotyledons structure developed, with a similar shape as zygotic mature embryos (Fig. 4c and d). Pre-cotyledonary embryos were formed in a high number, but only few of them developed towards the cotyledonary stage.
Proliferation and maturation of the ET. a Proliferation of the embryogenic tissue after subcultures on the proliferation medium. b Development of Pro-embryogenic masses on maturation media. c Production of cotyledons. d SE on the cotyledonary stage with a full shape. d Somatic embryo detached from the callus. e Germination of somatic embryo. Bars (a, b, c) = 1 mm, (d) = 2 cm
Cell lines Ab1, Ab2, Ab3 from tree IN7, and Ab4 from tree IN21 succeeded to reach the cotyledonary stage with 32% of total development in mature somatic embryos. Ab3 and Ab4 cell lines showed the highest maturation rate with 60% and 58%, respectively, followed by Ab2 (24%) and Ab1(12%). After 6 weeks from cotyledonary SE isolation and culture, solely 4,2% of mature somatic embryos showed an elongation of the hypocotyl and cotyledons turned green (Fig. 4d and e) with the appearance of a primordial root (Table 7).
Encapsulation and cryopreservation assay
Coated ET as artificial seeds from tree IN10 showed the ability to proliferate after encapsulation with a percentage of 80% (Fig. 5), while synthetic seeds from tree IN8 showed no proliferation. On the contrary, no callus proliferation was observed after the cryopreservation attempts.
The obtained results indicated the capacity of coated ET propagules to proliferate after their sowing, compared to those immersed in liquid nitrogen, where no proliferation was noted.
Discussion
Sicilian fir is characterized by abundant seed production every 3–4 years, similarly to other conifer species (Owens and Blake 1985). Generally, a high proportion of seeds were devoid of an embryo, which is the main cause of poor germination of A. nebrodensis seeds.
X-ray analysis was applied as a rapid technique; previously employed for the quality evaluation of seeds in forest species and viable embryos identification, such as in Pinus sylvestris (Simak et al. 1989; Sahlen et al. 1995) and Abies alba (Skrzyszewska, and Chłanda, 2009). Previous studies showed the effect of seed weight on the presence of embryos in mature seeds (Scialabba et al. 2009), but this method is laborious and time-consuming. X-ray based imaging, on the contrary, provides a non-destructive and high throughput method for seed quality evaluation, as initially discovered by Röntgen in 1895 (Glasser 1995). This technique was applied, for the first time, by Lundström (1903) to analyse coniferous tree seeds, in the pine cones. The utility of the X-ray method has been confirmed in this study where it simplified the experimental protocol process to induce the ET.
Abies species megagametophytes with immature embryos have been commonly used to achieve somatic embryogenesis (Salaj et al. 2020; Krajňáková et al. 2014). A. nebrodensis, immature cones were limited in quantity, and the selection of seeds containing megagametophytes was difficult. Mature embryos have been used as explants to induce ET in Picea abies and Abies alba × Abies cephalonica (Hazbuska-Przybyl and Bojarczuk 2008; Salajová and Salaj 2003). The present work reports the first successful somatic embryogenesis obtained from mature zygotic embryos of Abies nebrodensis (Fig. 6). The advantage of using mature zygotic embryos from such an endangered species, with a limited quantity of cones produced, is that mature seeds can be stored for a long period, but it may affect the callus induction rate.
Schematic representation of somatic embryogenesis for Abies nebrodensis. a Sicilian fir located in the Madonie Park. b Zygotic mature embryo c Initiation of ET d Proliferation of the ET. e and f Possibilities for cryopreservation of the germplasm. g Maturation of the ET and apparition of embryogenic structure. h Formation of full SE. i Embling germination
Our results, along with the first experiment, showed that SH basal salt medium was the best initiation medium, where IN7 showed the highest induction rate (40%). This basal salt medium was previously used by Gebhardt et al (1988) for A. alba ET induction from immature zygotic embryos.
Since conifer species frequently require the presence of both auxin and cytokinin to induce ET (Klimaszewska et al. 2002), the effect of some PGRs was tested. Our current investigations indicated that BAP applied alone on SH basal salt medium gave the best EC induction rates. 2,4-D gave no results if applied alone and caused a remarkable decrease of the EC induction rate when combined with BAP. These results fortified those obtained for Abies fraseri, A. normanniana, A. alba, and Greek fir, where cytokinin was the exclusive PGR used to induce and to maintain the ET (Salaj et al. 2020; Kim et al. 2009; Krajňáková et al. 2008; Nørgaard and Kgrostrup 1995).
In the third experiment, callus induction rates from different donor trees showed that IN8 presented the best induction rate (40%) when compared to the other tested donor trees. This result revealed a greater percentage for A. nebrodenesis, if compared to those reported by Von Arnold (1987) for Picea abies (8%), by Szczygieł and Kowalczyk (2001) for Abies alba (1,2–29,4% of callus initiation on mature embryos), by Salaj et al. (2020) for Abies alba Mill (0,83–13,33%) and by Gao et al. (2019) for Pinus korainesis (1,67%). This heterogeneity in callus induction rates could be explained and highly related to the donor tree age, maturity (Humánez et al. 2013; Niskanen et al. 2004) and cones storage period (our current experiment).
After ET induction, a proliferation and maintenance phases were carried out on the same initiation medium. Two to three weeks of subculture intervals gave satisfying results in maintaining the ET proliferation and confirming the importance of tissues sub-cultivations at regular intervals to improve the quality and the quantity of the proliferated ET (Klimaszweska et al. 2016; Gao et al. 2019). Long intervals between subcultures caused progressive tissue browning and necrotization (Salaj et al. 2019).
Furthermore, the presence of ABA (37,83 µM), PEG-4000 (8%) and maltose (40%) on SH medium were essentials to the success of somatic embryo maturation as well as the formation of late cotyledonary embryos in A. nebrodensis. These findings are consistent with prior studies where media enriched with different concentrations of ABA (75,66 µM and 37,83 µM) were the most frequently used to obtain mature somatic embryos from A. cilicica and A. cilicica × A. nordmanniana (Vookovà and Kormuťák 2003) and to promote cotyledonary SE formation, successively (Nørgaard 1997; Salajová and Salaj 2003).
For the genus Abies, SE was successfully regenerated from A. cilicica, A. nordmanniana and their hybrids (Vookovà and Kormu’tàk 2003), A. fraseri (Pullmann et al. 2016), A. numidica, A. concolor and A. alba (Vookovà and Kormu’tàk 2014). Zoglauer et al. (2012) reported that 80–90% of A. nordmanniana embryos with normal morphology were able to convert into emblings. Whereas species like A. lasiocarpa, A. balsamea, and A. cephalonica showed a limited regeneration. No relevant results were achieved for the germination assay conducted on the previously obtained somatic embryos of Abies nebrodensis, thus an optimized germination and acclimatization protocols are needed.
According to previous studies, somatic embryogenesis and tissue culture from the Sicilian fir have not been obtained until this present work (Krajňáková et al. 2014).
Conclusion
The induction and proliferation of embryogenic callus together with the maturation and germination of somatic embryos are key steps for large-scale propagation and long-term preservation of coniferous germplasm. In the presented work, an exclusive successful protocol for somatic embryogenis of the crititically endangered Abies nebrodensis, from mature zygotic embryos is described for the first time.
Employing in vitro cultures is difficult and laborious, but it showed the possibility to initiate somatic embryogenis as a new approach to propagate and conserve such a relict species. Investigations on SE induction from immature zygotic embryos were carried out but no relevant results were recorded. The reported findings of experiments suggest that the appropriate selection of donor trees, PGRs and basal salt media could help the maturation of somatic embryos into bipolar structures leading to emblings regeneration and acclimatization stage in the future.
Cryopreservation of embryogenic tissue could be an effective effective method for long-term maintenance. Artificial seeds of ET could offer a great advantage to improve the creation of the protocol for cryopreservation. A promising and encouraging result was obtained for encapsulated ET via sythetic seeds technology, where they showed the capacity to proliferate after encapulation. Conversely, the cryconservation of synthetic seeds needs further investigations to establish a complete cryopreservation protocol as well as for SE induction from immature zygotic embryos.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- ABA:
-
Abscisic acid
- AC:
-
Active charcoal
- BAP:
-
6-Benzylaminopurine
- DKW:
-
Driver Kuniyuki Walnut by Driver and Kuniyuki 1984
- EC:
-
Embryogenic callus
- NEC:
-
Non-embryogenic callus
- ET:
-
Embryogenic tissue
- h:
-
Hours
- min:
-
Minutes
- IN:
-
Identification number of the trees
- MS:
-
Murashige and Skoog 1962
- NAA:
-
1-Naphthalene acetic acid
- PEG:
-
Polyethylene glycol
- PGRs:
-
Plant growth regulators
- SH:
-
Schenk and Hilderbrandt 1972
- WPM:
-
Woody Plant Medium by Lloyd and McCown 1980
- PVS2:
-
Plant vitrification solution 2
References
Driver JA, Kuniyuki AH (1984) In vitro propagation of Paradox walnut rootstock. HortScience 19:507–509
Gao F, Peng C, Wang H, Shen H, Yang L (2019) Selection of culture conditions for callus induction and proliferation by somatic embryogenesis of Pinus koraiensis. J for Res 32:483–491
Gebhardt KH, Weisberger H, Fröhlich HJ (1988) In vitro germination and production of embryogenic callus from liquid suspension cultures of Abies alba. 4th International Conifer Tissue Culture Working Group, poster no. 17, Saskatoon, Canada
Glasser O (1995) WC roentgen and the discovery of the roentgen rays: history of radiology. AJR 165:1033–1040
Hakman I, Fowke LC, Von Arnold S, Eriksson T (1985) The development of somatic embryos in tissue cultures initiated from immature embryos of Picea abies (Norway spruce). Plant Sci 38:53–59
Hazubska-Przybył T, Wawrzyniak MK, Kijowska-Oberc J, Staszak AM, Ratajczak E (2022) Somatic Embryogenesis of Norway Spruce and Scots Pine: Possibility of Application in Modern Forestry. Forests 13:155
Hazubska-Przybyl T, Bojarczuk K (2008) Somatic embryogenesis of selected spruce species (Picea abies, P. omorika, P. pungens’ Glauca’and P. breweriana). Acta Soc Bot Pol 77:189–199
Humánez A, Blasco M, Brisa C, Segura J, Arrillaga I (2013) Somatic embryogenesis from different tissues of Spanish populations of maritime pine. Plant Cell Tissue Organ Cult 111:373–383
Isah T (2016) Induction of somatic embryogenesis in woody plants. Acta Physiol Plant 38:1–22
Kim YW, Newton R, Frampton J, Han KH (2009) Embryogenic tissue initiation and somatic embryogenesis in Fraser fir. In Vitro Cell Dev Biol Plant 45:400–406
Klimaszewska K, Hargreaves C, Lelu-Walter MA, Trontin JF (2016) Advances in conifer somatic embryogenesis since year 2000. In: Germanà MA, Lambardi M (eds) vitro embryogenesis in higher plants Press. Humana, New York, pp 131–166
Klimaszewska K, Cyr DR (2002) Conifer somatic embryogenesis: I. Development. Dendrobiology 48:41–49
Krajňáková J, Gömöry D, Häggman H (2008) Somatic embryogenesis in Greek fir. Can J for Res 38:760–769
Krajňáková J, Gömöry D, Häggman H (2014) Biotechnology tools for conservation of the biodiversity of European and Mediterranean Abies species. In: Ahuja MR, Ramawat KG (eds) Biotechnology and Biodiversity. Springer International Publishing, Berlin
Lelu-Walter MA, Thompson D, Harvengt L, Sanchez L, Toribio M, Pâques LE (2013) Somatic embryogenesis in forestry with a focus on Europe: state-of-the-art, benefits, challenges and future direction. Tree Genet Genomes 9:883–899
Loyola-Vargas VM, Ochoa-Alejo N (2016) Somatic embryogenesis. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Somatic embryogenesis fundamental aspects and applications. Springer, Berlin
Lundström AN 1903 Diskussionsinlagg rid For f. Skogsvard disk.mote a Robertsfors Arsskr. fran Foren. f. Skegavard i Norrland, Stockholm, pp 115
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Report 13:442–446
Micheli M, Standardi A (2016) From somatic embryo to synthetic seed in Citrus spp. through the encapsulation technology. In: Germanà MA, Lambardi M (eds) In vitro embryogenesis in higher plants Press. Newyork, New York, Humana, pp 515–522
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Niskanen AM, Lu J, Seitz S, Keinonen K, Von Weissenberg K, Pappinen A (2004) Effect of parent genotype on somatic embryogenesis in Scots pine (Pinus sylvestris). Tree Physiol 24:1259–1265
Nørgaard JV (1997) Somatic embryo maturation and plant regeneration in Abies nordmanniana LK. Plant Sci 124:211–221
Nørgaard JV, Krogstrup P (1995) Somatic embryogenesis in Abies spp. In: Mohan Jain S, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants III. Kluwer Academic Publishers, Dordrecth
Owens JN, Blake MD (1985) Forest tree seed production. In: Owens JN, Blake MD (eds) Canadian Forestry Service, Petawawa National Forestry Institute, Chalk River, Ontario. Information Report PI-X-53
Pasta S, Sala G, La Mantia T, Bondì C, Tinner W (2020) The past distribution of Abies nebrodensis (Lojac.) Mattei: results of a multidisciplinary study. Veg Hist Archaeobotany 29:357–371
Pullmann GS, Olson K, Fischer T, Egertsdotter U, Frampton J, Bucalo K (2016) Fraser fir somatic embryogenesis: high frequency initiation, maintenance, embryo development, germination, and cryopreservation. New for 47:453–480
Sahlen K, Bergsten U, Wiklund K (1995) Determination of viable and dead Scots pine seeds of different anatomical maturity after freezing using the IDX method. Seed Sci Res 23:405–414
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Salaj T, Klubicová K, Matusova R, Salaj J (2019) Somatic embryogenesis in selected conifer trees Pinus nigra Arn and Abies hybrids. Front Plant Sci 10:13
Salaj T, Klubicová K, Panis B, Swennen R, Salaj J (2020) Physiological and structural aspects of in vitro somatic embryogenesis in Abies alba Mill. Forests 11:1210
Salajová T, Salaj J (2003) Somatic embryo formation on mature Abies alba × Abies cephalonica zygotic embryo explants. Biol Plant 47:7–11
Salajová T, Jasik J, Kormutak A, Salaj J, Hakman I (1996) Embryogenic culture initiation and somatic embryo development in hybrid firs (Abies alba × Abies cephalonica and Abies alba × Abies numidica). Plant Cell Rep 15:527–553
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Plant Sci 50:199–204
Schuller A, Reuther G, Geier T (1989) Somatic embryogenesis from seed explants of Abies alba. Plant Cell Tissue Organ Cult 17:53–58
Scialabba A, Lombardo G, Schicchi R (2009) Seed mass variation and in vitro embryos culture of Abies nebrodensis (Lojac.) Mattei. In Biodiversity: Hotspots in the Mediterranean Area: species, communities and landscape level. 45th International congress SISV and FIP. Cagliari pp 307
Silva, JP (2008) LIFE and endangered plants: Conserving Europe's threatened flora. Office for Official Publications of the European Communities
Simak M, Bergsten U, Henriksson G (1989) Evaluation of ungerminated seeds at the end of germination test by radiography. Seed Sci Technol 17:361–369
Skrzyszewska K, Chłanda J (2009) A study on the variation of morphological characteristics of silver fir (Abies alba Mill.) seeds and their internal structure determined by X-ray radiography in the Beskid Sądecki and Beskid Niski Mountain ranges of the Carpathians (southern Poland). For Sci 55:403–414
Szczygieł K, Kowalczyk J (2001) Somatic embryogenesis of silver fir (Abies alba Mill.) – polish provenances. Proceedings of the 4th International Symposium In Vitro Culture and Horticultural Breeding. Tampere, Finland. Acta Hortic 560: 509–512
Thomas P (2017) Abies nebrodensis. The IUCN Red List of Threatened Species 2017. e.T30478A91164876. https://doi.org/10.2305/IUCN.UK.20172.RLTS.T30478A91164876.en. Accessed 15 Mar 2016
Von Arnold S (1987) Improved efficiency of somatic embryogenesis in mature embryos of Picea abies (L.) Karst. Plant Physiol 128:233–244
Vookovà B, Kormutak A (2003) Plantlet regeneration in Abies cilicica Carr. and Abies cilicica × Abies nordmanniana hybrid via somatic embryogenesis. Turk J Botany 27:71–76
Vookovà B, Kormuťák A (2007) Abies Biotechnology – Research and Development of Tissue Culture Techniques for Vegetative Propagation. Tree for Sci Biotech 1:39–46
Vookovà B, Kormutak A (2014) Study of Abies somatic embryogenesis and its application. Dendrobiology 71:149–157
Vookovà B, Gajdosova A, Matusova R (1997) Somatic embryogenesis in Abies × Abies alba and Abies alba × Abies nordmanniana hybrids. Biol Plant 40:523–530
Zoglauer K, Aurich C, Uehre P, Herrmann S (2012) Somatic embryogenesis in Abies nordmanniana: present status and future application In: Integrating vegetative propagation, biotechnologies and genetic improvement for tree production and sustainable forest management. Park YS, Bonga JM (eds) Proceedings of the IUFRO Working Party 2.09.02 conference. Brno Czech Republic, pp 196
Acknowledgements
We thank Prof. Rosario Schicchi (University of Palermo), Dr. Giuseppe Di Noto and Dr. Gaetano La Placa (University of Palermo) for providing the cones of Abies nebrodensis.
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement. This study was financially supported by LIFE project No. LIFE18 NAT/IT/000164 LIFE4FIR ‘Decisive in situ and ex situ conservation strategies to secure the critically endangered Sicilian Fir, Abies nebrodensis’, Coordinator: Dr. Roberto Danti.
Author information
Authors and Affiliations
Contributions
NJ: carried out the in vitro culture experiments, processed the experimental data and the statistical analysis, designed the figures, and wrote the first draft. EY: wrote the introduction, corrected the manuscript, and helped in the methods and results writing. WT: collaborated in a part of the in vitro experiments and gave advices. TI: collaborated in the X-Ray experiment and gave advices for the statistical analysis. CB and ML: are the responsible of the financial support for WT and TI fellowships, collaborated with the encapsulation and cryopreservation experiments and corrected the manuscript. MAG: is the responsible for Somatic Embryogenesis action, received the financial support for NJ fellowship gave the main conceptual ideas, reviewed the final version of the manuscript. All authors provided critical feedback and helped shape the research, analysis, manuscript, and approved the final submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jouini, N., Yahyaoui, E., Tarraf, W. et al. Somatic embryogenesis in Abies nebrodensis, an endangered Sicilian fir. Plant Cell Tiss Organ Cult 152, 393–404 (2023). https://doi.org/10.1007/s11240-022-02415-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02415-0