Abstract
The popularity of nanoparticles (NPs) is continuously increasing. To date, however, there has been little research on the application of NPs in plant cryopreservation, i.e. storage of tissues in liquid nitrogen (LN). The aim of this study is to analyze the effect and evaluate the usefulness of gold nanoparticles (AuNPs) in regard to cryobiology studies. In vitro-derived shoot tips of Lamprocapnos spectabilis ‘Valentine’ were cryopreserved with the encapsulation-vitrification protocol. Gold nanoparticles (at 10–30 ppm concentration; 13 nm in size) were added either into the preculture medium; to the protective bead matrix during encapsulation; or to the recovery medium after rewarming of samples. The control plants were produced from cryopreserved explants non-treated with nanoparticles or treated with colloid dispersion medium without NPs. A non-LN-treated standard was also considered. The influence of AuNPs on the cryopreservation efficiency was determined by evaluating the recovery rate of explants and their morphogenic response; the membrane stability index (MSI); the concentration of pigments in shoots; and the antioxidant enzymes activity. The genetic stability of the plant material was evaluated using Start Codon Targeted Polymorphism (SCoT) markers. It was found that 10 ppm of AuNPs added into the alginate bead matrix improved the recovery level of LN-derived shoot tips (70.0%) compared to the non-NPs-treated cryopreserved control (50.5%). On the other hand, the presence of nanoparticles in the recovery medium had a deleterious effect on the survival of explants. AuNPs usually had no impact on the MSI (73.9–85.9%), except for those added into the recovery medium at the concentration of 30 ppm (decline to 55.8%). All LN-derived shoots were shorter and contained less chlorophyll and carotenoids than the untreated standard. Moreover, the application of AuNPs affected the enzymatic activity in L. spectabilis. Minor genetic variation was found in 8.6% of plants if AuNPs were added either into the preculture medium (at 10 and 20 ppm) or to the alginate matrix (at 30 ppm). In conclusion, AuNPs added at a lower concentration (10 ppm) into the protective bead matrix can significantly improve the cryopreservation efficiency in L. spectabilis with no alternation in the DNA sequence.
Key message
This is the first study on the application of gold nanoparticles (AuNPs) in plant cryopreservation. Supplementation of alginate bead matrix with AuNPs improves the efficiency of cryopreservation in L. spectabilis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The protection of biodiversity is important for systematics research, species evolution understanding, and in breeding programs (Deliège and Neuteleers 2015). Cryopreservation, i.e. maintenance of biological material at the cryogenic temperature of liquid nitrogen (LN; − 196 °C), is currently considered the most effective long-term storage method. This approach has been applied to numerous plant species (Kulus and Zalewska 2014). Nonetheless, cryobiology is still a young science and requires more research.
A typical cryopreservation protocol is quite complex and requires careful optimization of several steps. The explant must be properly prepared for the storage at cryogenics to survive and maintain its recovery potential. Consequently, the plant material is exposed to hardening, dehydration, and rapid cooling/rewarming cycles. After storage in LN, the explants are again exposed to osmotic shock related to their rehydration. All of those steps may induce oxidative stress through the formation of free radicals, which may (micro)fracture the cell membrane system or affect the stability of plants (Zevallos et al. 2013b). Kulus and Abratowska (2017) described cryopreservation-induced-damage in the ultrastructure of shoot tips of Ajania pacifica (Nakai) Bremer et Humphries ‘Bengo’. Recent research demonstrated also several significant effects of cryopreservation recorded at the biochemical level of wild tomato (Solanum lycopersicum L.) plants, i.e. increased levels of peroxidase enzymatic and specific activities and cell wall-linked phenolics (Zevallos et al. 2013a). Kulus et al. (2019) reported, that cryopreservation by encapsulation-dehydration not only reduced the content of chlorophyll in the leaves of three chrysanthemum (Chrysanthemum × morifolium Ramat.) cultivars but also suppressed their vegetative growth and development. Nanoparticles (NPs) may significantly affect the effectiveness of long-term storage protocols and ought to be scrupulously tested.
The increasing interest in NPs results in their broad application in various disciplines (Tymoszuk and Miler 2019). Nanoparticles are defined as atomic or molecular aggregates of size less than 100 nm in at least one dimension. Due to their small size and accompanying unique physical and chemical properties, NPs can easily interact with the plant cell (Fayez et al. 2017). The most commonly manufactured NPs include carbon nanotubes, aluminum, copper, gold, iron, silica, silver, zinc, zinc oxide and titanium dioxide nanoparticles. Silver nanoparticles (AgNPs) are the best tested for plant research applications. It was found that AgNPs can affect the regeneration capacity of several plant species cultured in vitro (Tymoszuk and Miler 2019). Conversely, information on the application of gold nanoparticles (AuNPs) in plant studies is scarce and needs better exploration. Generally, there is a lack of comprehensive research demonstrating (cyto)genetic, biochemical, and phenotype effects of NPs application on plants, especially if applied for long-term storage.
A particularly interesting property of NPs is their high thermal conductivity (Li et al. 2005), which may be of great significance in cryobiology. To develop a successful cryopreservation protocol, it is imperative to achieve maximum cooling and rewarming pace of biological samples. By those means, the formation of lethal ice crystals at sub-zero temperatures is prevented. Accumulation of nanoparticles in plant tissues during the first step of a cryopreservation procedure, the so-called preculture, could significantly accelerate the thermodynamic events occurring during cooling and rewarming of biological material. Studies have demonstrated that thermal conductivity can be increased by > 20% when suspending nanoparticles in water (Zhang 1997). Nanoparticles can transport heat flux and cause thermal equilibrium with the environment within 10–200 ps (Hu and Hartland 2002). Therefore, the application of NPs could simplify and speed-up the cryopreservation protocol, compared to the droplet-vitrification, vacuum infiltration vitrification (VIV), or D- and V-cryoplate techniques (reviewed by Kulus 2019). Apart from increasing the thermodynamic effects, the addition of NPs into the protective bead matrix or post-rewarming recovery medium may positively affect the recovery capability of the explants, as reported with adventitious shoots regeneration in Streptocarpus × hybridus Voss. (Tymoszuk and Miler 2019). Supplementing zinc oxide nanoparticles (ZnONPs) to the cryopreservation medium was recently reported with men spermatozoa (Isaac et al. 2017). Indeed, this approach minimized the “freeze–thaw-induced damage” to the semen samples. There is also considerable attention focused on the application of nanomaterials in the PCR reaction in genetic analyses. It was found that AuNPs decrease the melting temperature (Tm) of the duplexes formed with perfectly matched and mismatched primers and increase the Tm difference between them (Lou and Zhang 2013). Due to “the excellent heat transfer property of the nanoparticles”, a dramatic enhancement in the PCR efficiency (by increasing the heating and cooling rates) was reported by Li et al. (2005) and Vanzha et al. (2016). To date, however, there is scarce information on the application of NPs in plant cryopreservation. Montalbán et al. (2018) used silver nanoparticles in the culture medium and in the preservation solution for deep-freeze storage (at − 80 °C) of embryogenic cell lines of Pinus radiata D. Don with high recovery level (above 75%). Ren et al. (2020) found that single-wall carbon nanotubes (SWCNTs) added to the plant vitrification solution (PVS) improve cell survival rate and reduce oxidative injury in cryopreservation of Agapanthus praecox Willd. embryogenic callus. On the other hand, there are no reports on the application of nanoparticles in cryostorage of meristems or entire shoot tips, which are the most popular explant types used in plant cryopreservation (Kulus and Zalewska 2014).
Lamprocapnos spectabilis (L.) Fukuhara (bleeding-heart) is a widely known herbaceous perennial, reproduced vegetatively by cuttings. The species is popular on the horticultural market (Frey and Moretti 2019). Moreover, it is a source of desirable genes, encoding a strong resistance to diseases and the ability to endure severe cold stress (Lee and Lee 2003). Due to the presence of numerous alkaloids, bleeding heart can be used in medicine, pharmacology, and cosmetic industry, fostering the development of new market opportunities (Petruczynik et al. 2019). On the other hand, the number of endemic populations of the species is limited and threatened with genetic erosion (Hammer and Khoshbakht 2005). Therefore, bleeding heart is a suitable model organism for researching the protection of biodiversity.
The aim of this study was to test, for the first time, the usefulness of gold nanoparticles in the cryopreservation of plant genetic resources. Our goal was to analyze the influence of AuNPs, applied at various concentrations during different steps of a cryopreservation procedure, on the development and widely-investigated stability of bleeding heart. The scientific hypothesis assumed that due to their favorable thermal properties, NPs may have a positive impact on the survivability of the explants stored in liquid nitrogen (LN). It is possible that their presence might affect the primary and secondary metabolism of the LN-recovered plants, manifested as a change in their enzymatic activity and/or metabolite profile. The genetic stability of the plants was evaluated using PCR-based genetic markers.
Materials and methods
Culture medium and physical conditions in the growth room
The MS (Murashige and Skoog 1962) medium, solidified with 0.8% agar (w/v; Biocorp, Warsaw, Poland), was used in the experiments. The pH was adjusted to 5.8 after adding all media components (Chempur, Piekary Śląskie, Poland), prior to autoclaving at 121 °C for 20 min and 0.1 MPa. The concentration of sucrose, AuNPs, and plant growth regulators (provided by Sigma-Aldrich, St. Louis, MO, USA) is given in the specific stages of the experiment.
The cultures were kept in the growth room at 24 ± 1 °C, under 16-h photoperiod conditions and photosynthetic photon flux density of approximately 25.0 µmol m−2 s−1, unless otherwise stated.
Detailed characteristics of nanoparticles
Gold nanoparticles were obtained from Nanoparticles Innovation NPIN s.c. (Łódź, Poland). According to the manufacturer's information, AuNPs were produced by the seeded-mediated growth method (Ranoszek-Soliwoda et al. 2020). Briefly, an aqueous solution of gold (III) chloride hydrate (94.25 g, 2.01·10–4% wt; Sigma-Aldrich, ≥ 49%) was heated to the boiling point under reflux and, next, an aqueous solution of sodium citrate (5.75 g, 0.877% wt; Sigma-Aldrich, ≥ 99.0%) was added to the reaction mixture. The solution was heated for 15 min under stirring, and then the colloid was cooled down to room temperature. The concentration of AuNPs in the resulting colloid was 100 ppm. The size and size distribution were measured by Scanning Transmission Electron Microscopy (STEM) (Nova NanoSEM 450, FEI™, Hillsboro, OR, USA), at accelerating voltage 30 kV, and reached 13 ± 3 nm (Fig. 1).
Biological material and multiplication of plants
In-vitro-derived plantlets of L. spectabilis (L.) Fukuhara ‘Valentine’, cloned via the single-node method in the MS medium devoid of plant growth regulators (PGRs-free), were used as the source of explants. Shoot tips with 2–4 young leaves covering the meristem (2.0–3.0 mm in length) were used in the cryopreservation experiments.
Experiment I: effect of AuNPs added into the preculture medium
Preculture
In vitro-derived single-node explants were cultured for 7 days in the MS medium with 9% sucrose (w/v), 4.65 μM (1.0 mg L−1) kinetin, and 10 µM (2.62 mg L−1) of abscisic acid (ABA). AuNPs at 10, 20, or 30 ppm were poured onto the culture medium immediately after explants inoculation, 2 mL per one culture jar. One culture jar with ten explants was considered a single repetition.
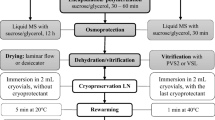
Encapsulation, dehydration, and LN storage
Shoot tips were excised and embedded for 10 min in 3% sodium-alginate (w/v) based on the MS medium salts, without calcium II chloride (CaCl2), supplemented with 9% sucrose. Then, the beads, 3–4 mm in diameter, were hardened in 0.1 M CaCl2 solution for 30 min. Firm beads were osmoprotected with the loading solution (2.0 M glycerol and 0.4 M sucrose) for 20 min. Next, the explants were dehydrated with Plant Vitrification Solution 3 (PVS3; 50% glycerol and 50% sucrose, w/v) for 150 min. Ten beads covered with PVS3 were placed in a 2.0 mL sterile cryovial and directly immersed in LN.
Rewarming and recovery
After storage overnight, the cryovials were taken from LN and rewarmed in a water bath at 39 ± 1 °C for 3 min. The PVS3 was removed from the vials with a pipette and the explants were rinsed with liquid MS medium with 1.2 M sucrose (for 30 min). Next, the still encapsulated shoot tips were inoculated on the MS recovery medium with 3% sucrose and 2.22 μM (0.5 mg L−1) 6-benzyladenine (BA) in a 90-mm Petri dish sealed with a parafilm. The cultures were kept in the growth room, in darkness. After 48 h, the explants were transferred to a 16-h photoperiod and kept at the light intensity of approximately 12.5 µmol m−2 s−1 for 5 days. Next, the shoot tips were transferred to initial lighting conditions. The experiment was repeated six times.
Experiment II: effect of AuNPs added into the protective bead matrix
Identical steps and parameters during the cryopreservation procedure were used in the second experiment. The only difference was that there were no NPs added into the preculture medium. Instead, the AuNPs; at 10 ppm, 20 ppm, or 30 ppm; were added into the alginate solution (step 2).
Experiment III: effect of AuNPs added into the recovery medium
The same steps and parameters during the cryopreservation protocol were used in the third experiment as in the other two, except AuNPs; at 10, 20, or 30 ppm; were poured onto the recovery medium after rewarming of samples (step 3).
Experimental controls and standard
Explants non-treated with nanoparticles at any stage of the cryopreservation procedure were considered as the negative control (named as ‘control’). Explants treated solely with colloid dispersion medium (used for the synthesis of NPs; i.e. sodium citrate 750 ppm and tannic acid 1 ppm), without AuNPs, added either into the preculture medium (step 1) or protective bead matrix (step 2), or recovery medium after rewarming of samples (step 3), served as positive controls (named as ‘0 ppm’). Moreover, a standard obtained from non-treated and non-cryopreserved nodal segments inoculated in a PGRs-free medium was included.
Evaluation of cryopreservation efficiency and biometrical analysis of plants
The share (%) of LN-derived explants producing shoots was evaluated after 30 days of recovery culture. Moreover, the number, length (mm), fresh (FW; mg), and dry weight (DW; %) of shoots were measured after 60 days. The DW was defined after drying the plant material in a laboratory oven at 105 °C for 3 h. The rooting efficiency (%) and number of roots per shoot were also included.
Evaluation of membrane stability index
The membrane stability index (MSI) was determined (%) by recording the electrical conductivity of fresh tissue leakages in double-distilled water (DDW) at 40 °C (initial conductivity) and 100 °C (final conductivity), following Dastborhan and Ghassemi-Golezani (2015) with minor modification. Six 100-mg tissue samples from each experimental object were taken in test tubes containing 10 mL of DDW. Conductivity was recorded using a conductivity meter after bringing the sample to 25 °C.
Biochemical array
The spectrophotometric analyses of pigment content and enzymatic activity were performed in the 9th and 10th week of culture using 100- and 200-mg fresh tissue samples, respectively, in six repetitions and a spectrophotometer SmartSpec PlusTM (BioRad, Hercules, CA, USA).
Analysis of pigment content
Chlorophylls and carotenoids were extracted from in vitro-grown shoots, as described by Lichtenthaler (1987) using 100% acetone (Chemia, Bydgoszcz, Poland). The spectrophotometric analysis of extracts was performed at specific wavelengths (λmax): for carotenoids at 470 nm, for chlorophyll a and b at 645 and 662 nm, respectively. The content of pigments was calculated per gram of fresh matter.
Determination of enzymatic activity
Samples taken from in vitro-grown shoots were homogenized in 100 mM phosphate buffer (pH 7.4) containing 1 mM EDTA, 1 mM dithiothreitol (DTT), and 2% polyvinylpyrrolidone (PVP) (all chemicals provided by Chemat, Gdańsk, Poland), according to Homaee and Ehsanpour (2016). The homogenates were centrifuged at 13,000×g for 20 min at 4 °C (Centrifuge MPW-260R, MPW MED INSTRUMENTS, Warsaw, Poland) and supernatants were used to determine the activities of antioxidant enzymes and protein content. The superoxide dismutase (SOD; EC 1.15.1.1) activity in shoots was determined as described by Giannopolitis and Ries (1977) by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium chloride (NBT). Catalase activity (CAT; EC 1.11.1.6) was defined by monitoring the disappearance of H2O2 according to the method by Anderson et al. (1995). The guaiacol peroxidase (GPOX; EC 1.11.1.7) and ascorbate peroxidase (APX; EC 1.11.1.11) activities were measured spectrophotometrically according to Maehly and Chance (1954) and Nakano and Asada (1981), respectively, with modifications described by Nowogórska and Patykowski (2015). Protein content was measured based on the Bradford method (Bradford 1976) with bovine serum albumin (BSA) as the standard. The spectrophotometric analysis of extracts was performed at specific wavelengths: for proteins at 595 nm, for SOD at 560 nm, for CAT at 240 nm, for GPOX and APX at 470 and 290 nm, respectively. The enzymatic activity U (μmol min−1) was calculated per 1 mg of protein. Since the minute is not an SI base unit of time, the enzyme unit U was converted to katal, with the assumption that 1 U = 1/60 μkat = 16.67 nkat.
Genetic stability evaluation
The genetic fidelity of shootlets, after 10 weeks of in vitro culture, was assessed using Start Codon Targeted Polymorphism (SCoT) (Collard and Mackill 2009). A total of 70 shoots (five from each experimental object, including the AuNPs-treated plants, positive and negative controls, as well as the genotype standard) were analyzed.
Total genomic DNA was isolated from fresh tissues using a FastPrep-24 5G bead beating grinder and lysis system (MP Biomedicals Irvine, CA, USA) and Genomic Mini AX Plant Spin kit (A&A Biotechnology, Gdynia, Poland), according to the manufacturer’s instruction.
A total of five SCoT primers were used in the PCR reaction. PCR was performed in a gradient BioRad C1000 Touch thermal cycler with heated cover (Bio-Rad, Hercules, CA, USA) in the 25-μL reaction solution. The composition of the reaction solution, PCR profiles, and electrophoretic separation of amplified DNA fragments were described in Kulus (2020a). Gel images were recorded using a GelDoc XR + Gel Photodocumentation System (Bio-Rad, Hercules, CA, USA) UV transilluminator with Image Lab 4.1 software. Molecular weights of the fragments were estimated using a 100–5000 bp DNA molecular marker (Gene Ruler TM Express DNA Ladder, Thermo Fisher Scientific, Waltham, MA, USA).
The banding patterns were recorded as 0–1 binary matrix, where “1” indicates the presence and “0” the absence of a given fragment followed by statistical analysis. For every primer tested, the numbers of monomorphic, polymorphic, and specific bands, as well as the polymorphism information content (PIC) were counted.
Statistical analysis
The experiments were set in a completely randomized design. A total of 840 explants were used. The arcsine square root transformation of percentage data was employed. The results were statistically analyzed with one-way ANOVA and Duncan’s post-hoc test (P ≤ 0.05) using Statistica 12.0 software (StatSoft, Cracow, Poland). GenAlex 6.5 software (Peakall and Smouse 2012) was used to count the genetic distance, perform the principal component analysis (PCoA) and run the analysis of molecular variance (AMOVA) with the assumption that the experimental objects (Table 1) are 14 separate populations. The dendrograms were created based on agglomerative hierarchical clustering (AHC) with the unweighted pair group average method (UPGMA) using Statistica 12.0.
Results
Recovery and biometric analysis of bleeding heart after cryostorage
All of the standard non-treated explants produced typical shoots (Table 1). As for the cryopreservation-derived shoot tips, the highest recovery level (70.0%) was reported after adding 10 ppm of AuNPs into the protective bead matrix. A high 61.7–62.4% recovery was also found after supplementing the preculture medium with 10 ppm AuNPs and the alginate matrix with 20 ppm AuNPs, although it was not significantly different from the NPs-free control (50.5%). Conversely, the addition of nanoparticles at the concentration exceeding 10 ppm into the recovery medium lead to a significant decline in the survival of explants (15.7–27.0%).
Non-treated explants produced fewer shoots (1.2) than most of the cryopreserved experimental objects (2.1–2.8), however, there was no impact of AuNPs on this parameter (Table 1). Cryopreservation affected the elongation of shoots, which were significantly shorter (15.6–33.4 mm, regardless of AuNPs treatment) than the standard plants (50.8 mm). The highest fresh weight of shoots (704.7 mg) was produced if AuNPs (20 ppm) were added into the alginate bead, which was significantly higher than in the non-treated standard object (190.2 mg) but not different from the NPs-free control (502.3 mg). On the other hand, the addition of 10 ppm AuNPs into the recovery medium resulted in the production of the highest dry weight of shoots (24.4%). However, elevating their concentration resulted in a decrease of DW (to even 9.3%) in all three experiments (Table 1).
All of the shoots produced from the non-treated explants produced adventitious roots (Table 1). In contrast, supplementation of the preculture or recovery media with AuNPs or even solely with the medium for their synthesis (0 ppm), resulted in a significant decrease of this parameter (4.8–51.7%). Only shoots developed from synthetic seeds fortified with nanoparticles at the concentration of 10–20 ppm did not differ in terms of rooting efficiency either from the control or the standard. A similar phenomenon was reported considering the number of roots regenerated by a single shoot (Table 1). It was also observed that the roots of the standard object were longer than in any other experimental combination (data not shown).
All cryopreservation-derived plants had a similar habit with no clear phenotypical alternations visible, regardless of NPs-treatment.
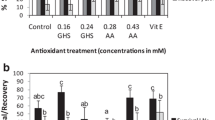
Only the shoots produced on the recovery medium with 30 ppm AuNPs had a significantly lower mean membrane stability index (55.8%) than those from the other experimental objects, which did not differ among each other (73.9–85.9%; Fig. 2).
Effect of AuNPs applied during the preculture (prec), encapsulation (enc), or recovery (rec) steps of the encapsulation-vitrification cryopreservation protocol on the membrane stability index (MSI) in Lamprocapnos spectabilis ‘Valentine’. Significant differences in values are determined by Duncan’s post hoc test (P < 0.05). Values with at least one same letter are not statistically different. Standard—non-treated explants cultured on the PGRs-free MS medium; control—cryopreserved but non-AuNPs-treated explants (negative control); 0 ppm—cryopreserved explants treated only with the colloid dispersion medium without AuNPs (positive control) at different steps of the cryopreservation procedure
Biochemical activity of bleeding heart after cryostorage
It was found that cryopreservation had a negative impact on the content of chlorophylls and carotenoids in the recovered shoots, which was reduced even 10–15 times compared to the untreated standard (Table 2). On the other hand, there were no differences between the control and experimental objects treated with AuNPs in terms of total chlorophyll and carotenoid concentration. The content of chlorophyll a, though, was significantly reduced if AuNPs were added either into the preculture medium at the highest 30 ppm concentration (50.6 µg g−1 FW) or to the recovery medium at 20–30 ppm (49.8–52.4 µg g−1 FW) compared to the control (88.6 µg g−1 FW). As for chlorophyll b, a higher concentration of this compound was found in shoots produced on the recovery medium supplemented with 30 ppm AuNPs (90.9 µg g−1 FW) than in the control (63.5 µg g−1 FW) and most NPs-treated plants. A higher ratio of chlorophyll a to b was found in the standard plants (2.4) compared to all LN-derived shoots (Table 2). The value of this parameter was > 1.0 in most bleeding hearts, except for the experimental objects in which AuNPs were added either into the preculture medium (at 30 ppm) or to the recovery medium (20–30 ppm). On the other hand, the highest chlorophylls to carotenoids ratio was found if 30 ppm of AuNPs were added into the preculture medium (10.9), which was significantly different from all other treatments, including the control (5.4) and standard plants (7.2). Plants developed from shoot tips embedded with a 30 ppm-AuNPs-fortified alginate matrix had the lowest value of this parameter (3.8).
NPs-treatment had a significant impact on the enzyme activity (Table 3). Higher activity of SOD in bleeding heart, compared with the untreated standard, was found if nanoparticles were added into the preculture medium, regardless of their concentration, or if 30 ppm AuNPs were added into the alginate bead matrix. However, only the experimental combination with the highest concentration of NPs (30 ppm) in the preculture medium, differed significantly from the control (333.0 and 187.0 nkat, respectively). On the other hand, the plants produced on the recovery medium fortified with any concentration of AuNPs had a markedly higher activity of APX (1.8–2.6 nkat) than the standard (0.6 nkat), although only the combinations with 20 and 30 ppm differed significantly from the control (1.1 nkat). All cryopreservation-derived shoots showed higher activity of GPOX (2.4–4.8 nkat) compared to the standard (0.7 nkat), but there was generally no impact of AuNPs on this parameter. Significantly higher activity of CAT was found solely if shoots were produced on the recovery medium with 30 ppm of AuNPs (58.8 nkat) compared to all other treatments, including the standard and control (6.1–21.7 nkat; Table 3).
Genetic stability of bleeding heart after cryostorage
A total of 2522 scorable bands, in the range from 150.1 to 2913.2 bp, were detected by five SCoT primers in 70 plants (Table 4). The highest number of loci (16) was amplified with primer no. 5, while five loci were found with primer no. 2. Primers no. 2 and 3 did not detect any polymorphism (0 PIC). On the other hand, 75.0% loci were polymorphic in four different genotypes according to primer no. 5 (Fig. 3). The highest PIC content was obtained with primer no. 4 (0.374). A total of six polymorphic plants (8.6%), obtained after adding 10–20 ppm of AuNPs into the preculture medium or 30 ppm into the bead matrix, were detected within the study. Four genotypes were confirmed as polymorphic by two primers, while the other two (obtained after adding 10 and 20 ppm AuNPs into the preculture medium) were detected by a single primer. No polymorphisms were found in the standard and control plants.
Example SCoT band profiles of Lamprocapnos spectabilis ‘Valentine’ received as a result of electrophoresis of the DNA amplification products obtained with primer no. 5. Outermost lanes (wm) are DNA bp weight markers, while inner lines represent plants treated with AuNPs (0–30 ppm) applied during the preculture (prec), encapsulation (enc), or recovery (rec) steps of the encapsulation-vitrification cryopreservation protocol. Standard—non-treated explants cultured on the PGRs-free MS medium; control—cryopreserved but non-AuNPs-treated explants (negative control); 0 ppm—cryopreserved explants treated only with the colloid dispersion medium without AuNPs (positive control) at different steps of the cryopreservation procedure. Arrows point to profiles that differ from the reference
UPGMA analysis of the SCoT marker system showed that the tested specimens were grouped into two clusters (Fig. 4). The highest genetic distance (3.6) was found for individual no. 1 from the experimental object with 10 ppm AuNPs in the preculture medium, which was placed in a separate cluster. The remaining plants were grouped into the second cluster, which was further divided into smaller primary, secondary and tertiary sub-clusters, with the largest one containing 64 individuals. The genetic distance between individuals in the second cluster ranged from 0.0 (in most cases) to 2.2.
Dendrogram based on the Euclid genetic distance matrix and UPGMA clustering presenting the relationships between analyzed Lamprocapnos spectabilis ‘Valentine’ plants treated with AuNPs (0–30 ppm) applied during the preculture (prec), encapsulation (enc), or recovery (rec) steps of the encapsulation-vitrification cryopreservation protocol, revealed by the Start Codon Targeted Polymorphism (SCoT) analysis. Standard—non-treated explants cultured on the PGRs-free MS medium; control—cryopreserved but non-AuNPs-treated explants (negative control); 0 ppm—cryopreserved explants treated only with the colloid dispersion medium without AuNPs (positive control) at different steps of the cryopreservation procedure
Findings of the UPGMA distribution were confirmed by the PCoA analysis, which arranged the studied plants in three main groupings of uneven size (Fig. 5). According to the AMOVA analysis of SCoT data, treatment with AuNPs had a significant influence on the occurrence of genetic variability in cryopreservation-derived bleeding heart ‘Valentine’, as 22% of the total variation was of interpopulation origin (Table 5).
Graph of principal coordinates analysis (PCoA) of Lamprocapnos spectabilis ‘Valentine’ plants treated with AuNPs (0–30 ppm) applied during the preculture (prec), encapsulation (enc), or recovery (rec) steps of the encapsulation-vitrification cryopreservation protocol, based on the Start Codon Targeted Polymorphism (SCoT) analysis. Standard—non-treated explants cultured on the PGRs-free MS medium; control—cryopreserved but non-AuNPs-treated explants (negative control); 0 ppm—cryopreserved explants treated only with the colloid dispersion medium without AuNPs (positive control) at different steps of the cryopreservation procedure. Plants representing the same band pattern as the predominant standard are collected within a single group named ‘monomorphic’
Discussion
Impact of AuNPs on the morphogenesis in LN-derived bleeding heart
The present study indicates a significant impact of gold nanoparticles on the recovery capability of LN-derived explants. Application of 10 ppm of AuNPs into the preculture medium or 10–20 ppm into the alginate bead matrix increased the cryopreservation efficiency by 11.2–19.5%. Similarly, silver and gold nanoparticles at low concentration (10 ppm) improved the micropropagation efficiency in Streptocarpus × hybridus (Tymoszuk and Miler 2019). This positive effect could result from the accelerated cooling and rewarming pace of the samples (Vanzha et al. 2016), which is crucial for cryopreservation success. On the other hand, higher AuNPs concentration had a negative impact on the shoot tip survival, especially if added into the recovery medium. This could be a result of cell damage or cell-cycle arrest not observed at lower concentrations (Ghosh et al. 2019). Similar morphogenesis-inhibitory-effects were reported in chrysanthemum internode explants treated with 20 ppm AgNPs (Tymoszuk and Kulus 2020). Application of 40 and 60 ppm AgNPs resulted in swelling, length reduction, and deformation of calli cells in two wheat (Triticum aestivum L.) cultivars (Barbasz et al. 2016). It is possible that after a longer period, nanoparticles in the recovery medium formed larger aggregates, which had a deleterious effect on the shoot and root development. This hypothesis is supported by a significant decline in the MSI value (55.8%) after 60 days of recovery culture on the MS medium with 30 ppm AuNPs. This underlines a shape- and concentration-dependent effect of nanoparticles reported also by other authors (Dietz and Herth 2011; Syu et al. 2014).
It was found that both shoots and roots of LN-recovered plants were shorter than those of the untreated standard. This is probably the effect of bead matrix which constitutes a physical barrier for the explant, impeding its development. The noticeable increase in shoot number after LN-storage, on the other hand, is the effect of a typical cell cryoinjury and activation of axillary buds, reported also in other species (Kulus et al. 2018; Kulus 2020a). The application of AuNPs does not affect this phenomenon. On the other hand, the presence of AuNPs in the culture medium had a negative impact on the rooting efficiency and root number in L. spectabilis, compared with the untreated standard. Our findings corroborate with those of Tymoszuk and Miler (2019), who reported that AgNPs at 10 and 30 ppm, inhibited adventitious rhizogenesis on chrysanthemum and Gerbera jamesonii Bolus explants. A possible explanation of this fact was suggested by Cvjetko et al. (2018), who found that that roots accumulate 30–100 times more nanoparticles than leaves. This effect, however, was not evident if AuNPs were added into the alginate bead matrix. This highlights the utility of nanoparticles in synthetic seed technology for the purpose of medium-term storage and propagation (Faisal and Alatar 2019), especially that nanoparticles are well-known for their antimicrobial properties (Huang et al. 2019).
The present research revealed also the impact of explant size and age on the cryopreservation efficiency in bleeding heart. In the study by Kulus (2020b), the recovery potential of smaller, 1–2-mm-long, shoot tips subjected to a similar encapsulation-vitrification protocol was higher compared to our control object by approximately 18%. This phenomenon was also described by Takagi et al. (1997) with Colocasia esculenta (L.) Schott. On the other hand, with Rosa × hybrida L., smaller explants (1–2 mm) were less efficient than larger ones (3–4 mm) with 2–4 leaf primordia after applying the droplet-vitrification technique (Halmagyi and Pinker 2006). The higher recovery potential of smaller explants could result from easier and more efficient dehydration of cells. Another possible explanation of the observed differences is the impact of season on the explant development in vitro, which was first described by Zalewska et al. (2011).
Impact of AuNPs on the biochemical events in LN-derived bleeding heart
The present study confirmed the negative impact of cryopreservation on the content of total chlorophylls and carotenoids in plants, reported previously when applying several other cryo-protocols to bleeding heart and other ornamental and medicinal plant species (Shibli et al. 1999; Kulus et al. 2019; Kulus 2020a). AuNPs did not prevent this phenomenon but, also, did not cause an even higher loss of those pigments compared to the NPs-free control. Only the content of chlorophyll a was significantly reduced if AuNPs were added at higher concentration to the preculture or recovery media, which marks this metabolite as even more labile than chlorophyll b. Consequently, the ratio of chlorophyll a to b and chlorophylls to carotenoids changed significantly in some experimental objects. NPs have an affinity for plant pigments, which might be the reason for those alternations (Mezacasa et al. 2020). A decline in chlorophyll a and total chlorophyll content was reported by Tymoszuk and Kulus (2020) in micropropagated chrysanthemum plants after treatment with 10 and 20 ppm AgNPs. In the study by Torres et al. (2018), gold nanoparticles at high concentrations caused a net decrease in the fluorescence ratio and quenching of chlorophyll fluorescence. As for the present study, only the content of chlorophyll b was higher than in the control samples if 30 ppm AuNPs were added into the recovery medium. This could be the result of enhanced photostability of chlorophyll b induced by gold nanoparticles (Barazzouk et al. 2012).
NPs-treatment affected also the activity of three antioxidant enzymes analyzed in the present study (Table 3). Interestingly, the action of individual enzymes varied depending on the treatment (pre- or post-cooling). The activity of SOD, APX, and CAT was significantly higher than in both untreated standard and NPs-free control if AuNPs were added at higher concentration into the preculture (SOD) or recovery media (APX, CAT). Nanomaterials can catalyze reduction–oxidation reactions in a plant cell and increase the production of reactive oxygen species (ROS) resulting in oxidative stress exertion and activation of antioxidative defense mechanism (Dietz and Herth 2011; Barbasz et al. 2016). To cope with stress conditions, plants have developed several enzymatic strategies to scavenge ROS produced in excess in cells (Dumont and Rivoal 2019). It is worth mentioning that GPOX seems to be the marker of dehydration and/or low-temperature stress in bleeding heart, as only this enzyme showed different activities in the standard and control plants, but was not affected by the presence and concentration of AuNPs.
Genetic stability of AuNPs- and LN-treated bleeding heart
In the current study, a total of six plants had an altered DNA sequence, i.e. 8.6% of all bleeding hearts, obtained after adding 10–20 ppm of AuNPs into the preculture medium or 30 ppm into the bead matrix. Among those six individuals, only one was detected by the UPGMA analysis as significantly different and placed in a separate cluster (Fig. 4). The obtained here low genomic variation is comparable to that observed in cryopreservation-derived Mentha × piperita L. (Martín et al. 2015), Hladnikia pastinacifolia Rchb. (Ciringer et al. 2018), and chrysanthemum (Kulus et al. 2019). The reason for those instabilities was found in the action of some cryoprotectants that, besides the dehydrating and stabilizing properties, demonstrate also cyto- and genotoxic effects. For example, a common cryoprotector-dimethyl sulfoxide (DMSO) can interact directly with the DNA by interfering with the metal cations, which are components of eukaryotic chromatin. Ethelene glycol (EG), on the other hand, is known to induce premature senescence of cells (Kulus and Zalewska 2014). Nonetheless, no polymorphisms were detected in the standard and control objects in the present study. This underlines at least a minor mutagenic effect of gold nanoparticles. The direct and indirect genotoxic effect of NPs has been reported previously (Ghosh et al. 2019). According to An and Jin (2012), nanoparticles have a high ability to bind to nucleic acids. They can alter the conformation of the DNA helix, and thus, change the orientation of nitrogenous bases in the DNA strand. The mutagenic effect of NPs can be also associated with the release of toxicants from their surface, such as metal ions or residues after synthesis (Barbasz et al. 2016). Tymoszuk and Kulus (2020) confirmed the mutagenic effect of silver nanoparticles in chrysanthemum. Halder et al. (2015) reported the genotoxic effect of copper nanoparticles already at a concentration of 3.2–6.4 ppm in Macrotyloma uniflorum (Lam.) Verdc.
Interestingly, in the present study, polymorphisms were detected only if AuNPs were used before storage of samples in LN; no polymorphisms were detected if nanoparticles were added into the post-rewarming recovery medium. This suggests that NPs-induced DNA damage is somewhat associated with dehydration and/or low-temperature stress. It is also worth mentioning that no mutation was detected in the most effective experimental object, i.e. 10 ppm AuNPs added to the alginate bead matrix. This is of utmost importance as while cryopreserving genetic resources, the emphasis has always been given to the genetic stability of the stored biological material. Perhaps lowering the concentration of AuNPs (to 5–8 ppm) would provide an even higher increase in explants survivability with no impact on their broadly understood stability.
Treatment of internode segments of chrysanthemum with 10 and 20 ppm AgNPs resulted in the creation of two new cultivars of this top ornamental plant species with rare burgundy and pinky-gold inflorescence colors (Tymoszuk and Kulus 2020). Jiang et al. (2013) reported that lipidoid-coated iron oxide nanoparticles can be applied for efficient DNA and siRNA delivery useful for plant breeding and genetic engineering purposes. Therefore, an increase in AuNPs content over 10 ppm is not recommended in cryopreservation of bleeding heart, but might be useful in mutation induction and breeding.
Conclusions
The present study sheds new light on the possible application of nanoparticles in plant studies, especially those related to biodiversity preservation. To the best of our knowledge, this is the first research on the application of AuNPs in cryopreservation of explants with differentiated tissues. The effect of gold nanoparticles is strongly connected with their concentration and method of plant treatment (addition of nanoparticles to the culture medium or the alginate bead matrix). AuNPs applicated at low concentration during the pre-cooling steps of the cryopreservation protocol, particularly encapsulation, have a beneficial impact on the survivability of the LN-stored shoot tips of bleeding heart. Supplementation of alginate bead matrix with 10 ppm of AuNPs enhances the recovery potential of LN-derived explants by approximately 20%, without altering their DNA sequence. Conversely, the addition of nanoparticles into the recovery medium after rewarming of samples has an unfavorable impact, resulting from the disruption of cell membranes and oxidative stress damage. The present study also confirmed minor genotoxic effects of AuNPs in suboptimal conditions, suggesting that when increased in concentration, they might be a valuable tool in breeding programs of bleeding heart. Future studies will focus on the utility of other nano-colloids, e.g. copper and titanium, in cryopreservation studies, particularly when added into the loading solution and PVS.
Data availability
Data available by e-mail on reasonable request.
References
An H, Jin B (2012) Prospects of nanoparticle-DNA binding and its implications in medical biotechnology. Biotechnol Adv 30:1721–1732. https://doi.org/10.1016/j.biotechadv.2012.03.007
Anderson MD, Prasad TK, Stewart CR (1995) Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol 109(4):1247–1257. https://doi.org/10.1104/pp.109.4.1247
Barazzouk S, Bekale LA, Hotchandani S (2012) Enhanced photostability of chlorophyll-a using gold nanoparticles as an efficient photoprotector. J Mat Chem 22(48):25316–25324. https://doi.org/10.1039/C2JM33681B
Barbasz A, Kreczmer B, Oćwieja M (2016) Effects of exposure of callus cells of two wheat varieties to silver nanoparticles and silver salt (AgNO3). Acta Physiol Plant 38:76. https://doi.org/10.1007/s11738-016-2092-z
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Ciringer T, Martín C, Šajna N, Kaligarič M, Ambrožič-Dolinšek J (2018) Cryopreservation of an endangered Hladnikia pastinacifolia Rchb. by shoot tip encapsulation-dehydration and encapsulation-vitrification. In Vitro Cell Dev Biol Plant 54(6):565–575. https://doi.org/10.1007/s11627-018-9917-yp
Collard BCY, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted merkers in plants. Plant Mol Biol Rep 27:86–93. https://doi.org/10.1007/s11105-008-0060-5
Cvjetko P, Zovko M, Peharec Štefanić P, Biba R, Tkalec M, Domijan AM, Vinković Vrček I, Letofsky-Papst I, Šikić S, Balen B (2018) Phytotoxic effects of silver nanoparticles in tobacco plants. Environ Sci Pollut Res 25:5590–5602. https://doi.org/10.1007/s11356-017-0928-8
Dastborhan S, Ghassemi-Golezani K (2015) Influence of seed priming and water stress on selected physiological traits of borage. Folia Hort 27(2):151–159. https://doi.org/10.1515/fhort-2015-0025
Deliège G, Neuteleers S (2015) Should biodiversity be useful? Scope and limits of ecosystem services as an argument for biodiversity conservation. Env Val 24(2):165–182. https://doi.org/10.3197/096327114X13947900181275
Dietz KJ, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16(1):582–589. https://doi.org/10.1016/j.tplants.2011.08.003
Dumont S, Rivoal J (2019) Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front Plant Sci 10:166. https://doi.org/10.3389/fpls.2019.00166
Faisal M, Alatar M (2019) Synthetic seeds: germplasm regeneration, preservation and prospects. Springer, Cham
Fayez KA, El-Deeb BA, Mostafa NY (2017) Toxicity of biosynthetic silver nanoparticles on the growth, cell ultrastructure and physiological activities of barley plant. Acta Physiol Plant 39:155. https://doi.org/10.1007/s11738-017-2452-3
Frey D, Moretti M (2019) A comprehensive dataset on cultivated and spontaneously growing vascular plants in urban gardens. Data Brief 25:103982. https://doi.org/10.1016/j.dib.2019.103982
Ghosh M, Ghosh I, Godderis L, Hoet P, Mukherjee A (2019) Genotoxicity of engineered nanoparticles in higher plants. Mut Res Genet Toxicol Environ Mut 842:132–145. https://doi.org/10.1016/j.mrgentox.2019.01.002
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Halder S, Mandal A, Das D, Chattopadhyay AP, Datta AK (2015) Copper nanoparticle induced macromutation in Macrotyloma uniflorum (Lam.) Verdc. (Leguminosae): a pioneer report. Genet Resourc Crop Evol 62:165–175. https://doi.org/10.1508/cytologia.82.267
Halmagyi A, Pinker I (2006) Plant regeneration from Rosa shoot tips cryopreserved by a combined droplet vitrification method. Plant Cell Tissue Organ Cult 84(2):100129–100137. https://doi.org/10.1007/s11240-005-9012-z
Hammer K, Khoshbakht K (2005) Towards a “red list” for crop plant species. Genet Res Crop Evol 52:249–265. https://doi.org/10.1007/s10722-004-7550-6
Homaee MB, Ehsanpour AA (2016) Silver nanoparticles and silver ions: oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic Environ Biotechnol 57(6):544–553. https://doi.org/10.1007/s13580-016-0083-z
Hu M, Hartland GV (2002) Heat dissipation for Au particles in aqueous solution: relaxation time versus size. J Phys Chem B 106(28):7029–7033. https://doi.org/10.1021/jp020581
Huang Q, Luo A, Jiang L, Zhou Y, Yang Y, Liu Q, Zhang C (2019) Disinfection efficacy of green synthesized gold nanoparticles for medical disinfection applications. Afr Health Sci 19(1):1441–1448. https://doi.org/10.4314/ahs.v19i1.17
Isaac AV, Kumari S, Nair R, Urs DR, Salian SR, Kalthur G, Adiga SK, Manikkath J, Mutalik S, Sachdev D, Pasricha R (2017) Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2017.10.112
Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG (2013) Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett 13:1059–1064. https://doi.org/10.1021/nl304287a
Kulus D (2019) Managing plant genetic resources using low and ultra-low temperature storage: a case study of tomato. Biodiv Conserv 28(5):1003–1027. https://doi.org/10.1007/s10531-019-01710-1
Kulus D (2020a) Shoot tip cryopreservation of Lamprocapnos spectabilis (L.) Fukuhara using different approaches and evaluation of stability on the molecular, biochemical, and plant architecture levels. Int J Mol Sci 21:3901. https://doi.org/10.3390/ijms21113901
Kulus D (2020b) Effect of bead composition, PVS type, and recovery medium in cryopreservation of bleeding heart ‘Valentine’: preliminary study. Agronomy 10:891. https://doi.org/10.3390/agronomy10060891
Kulus D, Abratowska A (2017) CRYOconservation of Ajania pacifica (Nakai) Bremer et Humphries via encapsulation-dehydration technique. CryoLett 38(5):387–398
Kulus D, Abratowska A, Mikuła A (2018) Morphogenetic response of shoot tips to cryopreservation by encapsulation-dehydration in a solid mutant and periclinal chimeras of Chrysanthemum × grandiflorum/Ramat./Kitam. Acta Physiol Plant 40:18. https://doi.org/10.1007/s11738-017-2593-4
Kulus D, Rewers M, Serocka M, Mikuła A (2019) Cryopreservation by encapsulation-dehydration affects the vegetative growth of chrysanthemum but does not disturb its chimeric structure. Plant Cell Tissue Organ Cult 138(1):153–166. https://doi.org/10.1007/s11240-019-01614-6
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species: a review. Sci Hortic 168:88–107. https://doi.org/10.1016/j.scienta.2014.01.014
Lee KP, Lee DW (2003) Somatic embryogenesis and plant regeneration from seeds of wild Dicentra spectabilis (L.) Lem. Plant Cell Rep 22:105–109. https://doi.org/10.1007/s00299-003-0642-5
Li M, Lin Y-C, Wu C-C, Liu H-S (2005) Enhancing the efficiency of a PCR using gold nanoparticles. Nucleic Acids Res 33(21):e184. https://doi.org/10.1093/nar/gni183
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lou X, Zhang Y (2013) Mechanism studies on nanoPCR and applications of gold nanoparticles in genetic analysis. ACS Appl Mat Interfaces 105(13):6276–6284. https://doi.org/10.1021/am4013209
Maehly AC, Chance B (1954) The assay of catalases and peroxidases. In: Glick D (ed) Methods of biochemical analysis. Willey, New York, pp 357–425
Martín C, Kremer C, González I, González-Benito ME (2015) Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tissue Organ Cult 122:185–195. https://doi.org/10.1007/s11240-015-0760-0
Mezacasa AV, Queiroz AM, Graciano DE, Pontes MS, Santiago EF, Oliveira IP, Lopez AJ, Casagrande GA, Scherer MD, dos Reis DD, Oliveira SL, Caires ARL (2020) Effects of gold nanoparticles on photophysical behaviour of chlorophyll and pheophytin. J Photochem Photobiol A 389:112252. https://doi.org/10.1016/j.jphotochem.2019.112252
Montalbán IA, Olarieta AC, Casillas-Figueroa F, Arellano-García ME, Chavez-Santoscoy AR, Pestryakov A, Bogdanchikova N, Moncalean P (2018) Simplified method to store embryogenic cells: silver nanoparticles and cryoprotectors elimination effect. Crybiol 85:134. https://doi.org/10.1016/j.cryobiol.2018.10.067
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nowogórska A, Patykowski J (2015) Selected reactive oxygen species and antioxidany enzymes in common bean after Pseudomonas syringae pv. phaseolicola and Botrytis cinerea infection. Acta Physiol Plant 37:1725. https://doi.org/10.1007/s11738-014-1725-3
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28(19):2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Petruczynik A, Plech T, Tuzimski T, Misiurek J, Kaproń B, Misiurek D, Szultka-Młyńska M, Buszewski B, Waksmundzka-Hajnos M (2019) Determination of selected isoquinoline alkaloids from Mahonia aquifolia; Meconopsis cambrica; Corydalis lutea; Dicentra spectabilis; Fumaria officinalis; Macleaya cordata extracts by HPLC-DAD and comparison of their cytotoxic activity. Toxins 11:575. https://doi.org/10.3390/toxins11100575
Ranoszek-Soliwoda K, Czechowska E, Tomaszewska E, Pudlarz A, Szemraj J, Celichowski G, Grobelny J (2020) Differences in corona formation of catalase immobilised on gold and silver nanoparticles. Colloids Surf A 600:125003. https://doi.org/10.1016/j.colsurfa.2020.125003
Ren L, Deng S, Chu Y, Zhang Y, Zhao H, Chen H, Zhang D (2020) Single-wall carbon nanotubes improve cell survival rate and reduce oxidative injury in cryopreservation of Agapanthus praecox embryogenic callus. Plant Meth 16(130):1–12. https://doi.org/10.1186/s13007-020-00674-6
Shibli RA, Smith MAL, Shatnawi MA (1999) Pigment recovery from encapsulated-dehydrated Vaccinium pahalae (ohelo) cryopreserved cells. Plant Cell Tissue Organ Cult 55:119–123. https://doi.org/10.1023/A:1006276205723
Syu YY, Hung JH, Chen JC, Chuang HW (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64. https://doi.org/10.1016/j.plaphy.2014.07.010
Takagi H, Tien Thinh NT, Islam OM, Senboku T, Sakai A (1997) Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Rep 16:594–599. https://doi.org/10.1007/BF01275498
Torres R, Diz VE, Lagorio MG (2018) Effects of gold nanoparticles on the photophysical and photosynthetic parameters of leaves and chloroplasts. Photochem Photobiol Sci 17:505–516. https://doi.org/10.1039/C8PP00067K
Tymoszuk A, Kulus D (2020) Silver nanoparticles induce genetic, biochemical, and phenotype variation in chrysanthemum. Plant Cell Tissue Organ Cult 143:331–344. https://doi.org/10.1007/s11240-020-01920-4
Tymoszuk A, Miler N (2019) Silver and gold nanoparticles impact on in vitro adventitious organogenesis in chrysanthemum, gerbera and Cape Primrose. Sci Hortic 257:108766. https://doi.org/10.1016/j.scienta.2019.108766
Vanzha E, Pylaev T, Khanadeev V, Konnova S, Fedorova V, Khlebtsov N (2016) Gold nanoparticle-assisted polymerase chain reaction: effects of surface ligands, nanoparticle shape and material. RSC Adv 6:110146–110154. https://doi.org/10.1039/C6RA20472D
Zalewska M, Lema-Rumińska J, Miler N, Gruszka M, Dąbal W (2011) Induction of adventitious shoot regeneration in chrysanthemum as affected by the season. In Vitro Cell Dev Biol Plant 47:375–378. https://doi.org/10.1007/s11627-010-9330-7
Zevallos B, Cejas I, Rodríguez RC, Yabor L, Aragón C, González J, Engelmann F, Martínez ME, Lorenzo JC (2013a) Biochemical characterization of Ecuadorian wild Solanum lycopersicum Mill. plants produced from non-cryopreserved and cryopreserved seeds. CryoLett 37(4):413–421
Zevallos B, Cejas I, Valle B, Yabor L, Aragón C, González J, Engelmann F, Martínez ME, Lorenzo JC (2013b) Short-term liquid nitrogen storage of wild tomato (Solanum lycopersicum Mill.) seeds modifies the levels of phenolics in 7 day-old seedlings. Sci Hortic 160:264–267. https://doi.org/10.1016/j.scienta.2013.06.002
Zhang JZ (1997) Ultrafast studies of electron dynamics in semiconductor and metal colloidal nanoparticles: effects of size and surface. Accounts Chem Res 30(10):423–429. https://doi.org/10.1021/ar960178j
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, DK. Data curation, DK and AT. Formal analysis, DK and AT. Investigation, DK and AT. Methodology, DK and AT. Project Administration, DK Resources, DK. Supervision, DK. Validation, DK. Visualization, DK. Writing-original draft preparation, D.K. Writing-review and editing, DK and AT. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Both authors declare that they have no conflict of interest.
Additional information
Communicated by Qiao-Chun Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kulus, D., Tymoszuk, A. Gold nanoparticles affect the cryopreservation efficiency of in vitro-derived shoot tips of bleeding heart. Plant Cell Tiss Organ Cult 146, 297–311 (2021). https://doi.org/10.1007/s11240-021-02069-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02069-4