Abstract
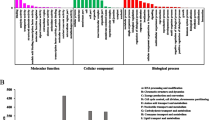
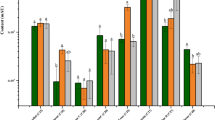
Atractylodes lancea: (Thunb.) DC. is an endangered traditional Chinese medicinal herb with broad pharmacological activity, reflected by an abundance of sesquiterpenoids. However, the biosynthesis of these sesquiterpenoids is largely unknown due to a lack of genetic information. To understand sesquiterpenoid distribution in A. lancea, tissue-specific sesquiterpenoids accumulation patterns during different growth periods of plantlets were analyzed via gas chromatography (n = 3). Transcriptomic analyses were furthermore performed via RNA-Seq in an attempt to reveal the molecular mechanism regulating sesquiterpenoids formation in leaves, stems and roots. 85,170 unigenes with an average sequence length of 768 bp were acquired. Via analysis of differentially expressed genes, 857 unigenes were identified to be involved in sesquiterpenoids formation. Consequently, a putative sesquiterpenoid biosynthetic pathway was proposed, including mevalonate, 2-C-methyl-d-erythritol-4-phosphate, sesquiterpene synthase, cytochrome P450, and the peroxisome pathway. Moreover, the relationship between gene expression and sesquiterpenoid production in different growth periods of leaves, stems, and roots was analyzed based on Quantitative Real-Time PCR (n = 3) and gas chromatography data. In this study, the sesquiterpenoid distribution in different tissues of sterile tissue culture plantlets of A. lancea was systematically investigated via metabolite quantitative and transcriptomics analysis. This study provides a central theoretical basis for the manipulation of sesquiterpenoid biosynthesis in A. lancea.









Similar content being viewed by others
Abbreviations
- A. lancea :
-
Atractylodes lancea (Thunb.) DC.
- GC:
-
Gas chromatography
- MS:
-
Mass spectrometry
- L. canum:
-
Leucosceptrum canum
- MS:
-
Murashige and Skoog
- NR:
-
Non-redundant
- NT:
-
Nucleotide
- COG:
-
Classification of Orthologous Group
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- GO:
-
Gene ontology
- FPKM:
-
Fragments per kilobase of transcript per million fragments mapped
- DEGs:
-
Differentially expressed genes
- FDR:
-
False discovery rate
- A. annua :
-
Artemisia annua
- qRT-PCR:
-
Quantitative Real-Time PCR
- MVA:
-
Mevalonate
- MEP:
-
2-C-methyl-d-erythritol-4-phosphate
- IPP:
-
Isopentenyl diphosphate
- DMAPP:
-
Dimethylallyl diphosphate
- HMGCR:
-
3-Hydroxy-3-methylglutaryl CoA synthase
- DXR:
-
1-Deoxy-d-xylulose-5-phosphate reductoisomerase
- IDI:
-
Isopentenyl diphosphate isomerase
- FDPS:
-
Farnesyl diphosphate synthase
- FS:
-
Farnesene synthase
- GAS:
-
Germacrene A synthase
- GDS:
-
Germacrene D synthase
- CPS:
-
β-Caryophyllene synthase
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
References
Ahmed S, Zhan C, Yang Y, Wang X, Yang T, Zhao Z, Zhang Q, Li X, Hu X (2016) The transcript profile of a traditional Chinese medicine, Atractylodes lancea, revealing its sesquiterpenoid biosynthesis of the major active components. PLoS One 11:0151975
Bryant L, Flatley B, Patole C, Brown G, Cramer R (2015) Proteomic analysis of Artemisia annua—towards elucidating the biosynthetic pathways of the antimalarial pro-drug artemisinin. BMC Plant Biol 15:175
Chen Q, Li P, Yang H, Li X, Zhu J, Chen F (2009) Identification of volatile compounds of Atractylode lancea rhizoma using supercritical fluid extraction and GC-MS. J Sep Sci 32:3152–3156
Chen F, Ren C, Zhou T, Wei Y, Dai C (2016) A novel exopolysaccharide elicitor from endophytic fungus Gilmaniella sp. AL12 on volatile oils accumulation in Atractylodes lancea. Sci Rep 6:34735
Duan Y, Zhu B, Shu S, Li Z, Wang M (2015) Karyotypes and fish detection of 5 S and 45 S rDNA loci in Chinese medicinal plant Atractylodes lancea subsp. luotianensis: cytological evidence for the new taxonomic unit. Pak J Bot 47:103–107
Grabherr MG, Haas BJ, Yassour M et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Gu W, Cao J, Zhang Y, Li D (2008) Wildlife tending of endangered herbal plant Atractylodes lancea (Thunb.) DC. J. Nanjing Univ Tradit. Chin Med 24:248–250 (Chinese)
Guo J, Zhou Y, Hillwig ML, Shen Y, Yang L, Wang Y, Zhang X, Liu W, Peters RJ, Chen X, Zhao Z, Huang L (2013) CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc Natl Acad Sci USA 110:12108–12113
Hale V, Keasling J, Renninger N, Diagana TT (2007) Microbially derived artemisinin: a biotechnology solution to the global problem of access to affordable antimalarial drugs. Am J Trop Med Hyg 77:198–202
Hara KY, Araki M, Okai N, Wakai S, Hasunuma T, Kondo A (2014) Development of bio-based fine chemical production through synthetic bioengineering. Microb Cell Fact 13:173
Hasada K, Yoshida T, Yamazaki T, Sugimoto N, Nishimura T, Nagatsu A, Mizukami H (2010) Quantitative determination of atractylon in Atractylodis rhizoma and Atractylodis lanceae rhizoma by 1H-NMR spectroscopy. J Nat Med 64:161–166
Hiraoka N (1995) Intra-plant distribution of essential oil components and oil accumulation issues in Atractylodes lancea. Nat Med 49:168–171
Hu S, Feng X, Ji L, Nie S (2000) Atractylodes lancea and its geo-varieties. Chin Tradit Herbal. Drugs 31:781–784 (Chinese)
Huang Q, Huang X, Deng J, Liu H, Liu Y, Yu K, Huang B (2016) Differential gene expression between leaf and rhizome in Atractylodes lancea: a comparative transcriptome analysis. Front Plant Sci 7:348
Kling J (2016) Protecting medicine’s wild pharmacy. Nat Plants 2:16064
Kraker JW, Franssen MCR, Dalm MCF, Groot A, Bouwmeester HJ (2001) Biosynthesis of germacrene A carboxylic acid in chicory roots. Demonstration of a cytochrome P450 (+)-germacrene A hydroxylase and NADP+-dependent sesquiterpenoid dehydrogenase(s) involved in sesquiterpene lactone biosynthesis. Plant Physiol 125:1930–1940
Kraker JW, Franssen MCR, Joerink M, Groot A, Bouwmeester HJ (2002) Biosynthesis of costunolide, dihydrocostunolide, and leucodin. Demonstration of cytochrome P450-catalyzed formation of the lactone ring present in sesquiterpene lactones of chicory. Plant Physiol 129:257–268
Lee GY, Kim HM, Ma SH, Park SH, Joung YH, Yun CH (2014) Heterologous expression and functional characterization of the NADPH-cytochrome P450 reductase from Capsicum annuum. Plant Physiol Biochem 82:116–122
Liu W, Gong T, Zhu P (2016a) Advances in exploring alternative Taxol sources. RSC Adv 6:48800–48809
Liu Y, Luo S, Schmidt A, Wang G, Sun G, Grant M, Kuang C, Yang M, Jing S, Li C, Schneider B, Gershenzon J, Li S (2016b) A geranylfarnesyl diphosphate synthase provides the precursor for sesterterpenoid (C25) formation in the glandular trichomes of the mint species Leucosceptrum canum. Plant Cell 28:804–822
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using Real-time quantitative PCR and the 2–∆∆Ct method. Methods 25:402–408
Lopez-Huertas E, Charlton WL, Johnson B, Graham IA, Baker A (2000) Stress induces peroxisome biogenesis genes. EMBO J 19:6770–6777
Lvcker J, Bowen P, Bohlmann J (2004) Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (–)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 65:2649–2659
May B, Lange M, Wüst M (2013) Biosynthesis of sesquiterpenes in grape berry exocarp of Vitis vinifera L.: Evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol. Phytochemistry 95:135–144
Mercke P, Crock J, Croteau R, Brodelius PE (1999) Cloning, expression, and characterization of epi-cedrol synthase, a sesquiterpene cyclase from Artemisia annua L. Arch Biochem Biophys 369:213–222
Mintz-Oron S, Meir S, Malitsky S, Ruppin E, Aharoni A, Shlomi T (2012) Reconstruction of Arabidopsis metabolic network models accounting for subcellular compartmentalization and tissue-specificity. Proc Natl Acad Sci USA 109:339–344
Peng H, Yuan Q, Li Q, Huang L (2012) Molecular systematics of genus Atractylodes (Compositae, Cardueae): evidence from internal transcribed spacer (ITS) and trnL-F sequences. Int J Mol Sci 13:14623–14633
Rasulov B, Talts E, Kännaste A, Niinemets U (2015) Bisphosphonate inhibitors reveal a large elasticity of plastidic isoprenoid synthesis pathway in isoprene-emitting hybrid aspen. Plant Physiol 168:532–548
Ren C, Dai C (2012) Jasmonic acid is involved in the signaling pathway for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. BMC Plant Biol 12:128
Seo M, Kim S, Kang T, Rim H, Jeong H, Um J, Hong S, Kim H (2011) The regulatory mechanism of β-eudesmol is through the suppression of caspase-1 activation in mast cell-mediated inflammatory response. Immunopharmacol Immunotoxicol 33:178–185
Takase H, Sasaki K, Shinmori H, Shinohara A, Mochizuki C, Kobayashi H, Ikoma G, Saito H, Matsuo H, Suzuki S, Takata R (2016) Cytochrome P450 CYP71BE5 in grapevine (Vitis vinifera) catalyzes the formation of the spicy aroma compound (–)-rotundone. J Exp Bot 67:787–798
Wang Y, Dai C, Zhao Y, Peng Y (2011) Fungal endophyte-induced volatile oil accumulation in Atractylodes lancea plantlets is mediated by nitric oxide, salicylic acid and hydrogen peroxide. Process Biochem 46:730–735
Wang X, Yang B, Ren C, Wang H, Wang J, Dai C (2015) Involvement of abscisic acid and salicylic acid in signal cascade regulating bacterial endophyte-induced volatile oil biosynthesis in plantlets of Atractylodes lancea. Physiol Plantarum 153:30–42
Weitzel C, Simonsen H (2015) Cytochrome P450-enzymes involved in the biosynthesis of mono- and sesquiterpenes. Phytochem Rev 14:7–24
Wurtzel ET, Kutchan TM (2016) Plant metabolism, the diverse chemistry set of the future. Science 353:1232–1236
Xu K, Jiang J, Feng Z, Yang Y, Li L, Zang C, Zhang P (2016) Bioactive sesquiterpenoid and polyacetylene glycosides from Atractylodes lancea. J Nat Prod 79:1567–1575
Yang L, Yang C, Li C, Zhao Q, Liu L, Fang X, Chen X (2016) Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci Bull 61:3–17
Yuan J, Zhou J, Li X, Dai C (2016) The primary mechanism of endophytic fungus Gilmaniella sp. AL12 promotion of plant growth and sesquiterpenoid accumulation in Atractylodes lancea. Plant Cell Tissue Org Cult 125:571–584
Zhang W, Ouyang Z, Zhao M, Wei Y, Peng H, Wang Q, Guo L (2015) The influences of inorganic elements in soil on the development of famous - region Atractylodes lancea (Thunb.) DC. Pharmacogn Mag 42:337–344
Zhao Q, Guo L, Huang L, Zhang X (2008) Relationship between essential oil variation and genetic variation of Atractylodes lancea. Resources Sci 30:765–769 (in Chinese)
Zhou J, Li X, Zheng J, Dai C (2016a) Volatiles released by endophytic Pseudomonas fluorescens promoting the growth and volatile oil accumulation in Atractylodes lancea. Plant Physiol Biochem 101:132–140
Zhou J, Yuan J, Li X, Ning Y, Dai C (2016b) Endophytic bacterium-triggered reactive oxygen species directly increase oxygenous sesquiterpenoid content and diversity in Atractylodes lancea. Appl Environ Microbiol 82:1577–1585
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21406119 and 31070443), Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (14KJB180009), Integration of Production and Research Projects of Nanjing Science and Technology Commission (201306019), and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author contributions
Chuan-Chao Dai and Fei Chen conceived and designed the experiments. Fei Chen, Yu-Xiao Wei, Jing-Min Zhang, Xiu-Mei Sang performed the experiments. Fei Chen analyzed the data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, F., Wei, YX., Zhang, JM. et al. Transcriptomics analysis investigates sesquiterpenoids accumulation pattern in different tissues of Atractylodes lancea (Thunb.) DC. plantlet. Plant Cell Tiss Organ Cult 130, 73–90 (2017). https://doi.org/10.1007/s11240-017-1205-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1205-8