Abstract
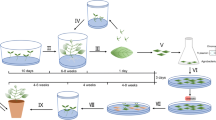
In mature citrus transformation, the nptII gene is most commonly used for selection and it is confounded by the high number of non-transformed, escaped shoots that develop on semi-solid kanamycin selection medium, even at high concentrations. Selection in liquid medium with kanamycin in temporary immersion bioreactors might provide a better means of distinguishing between transformed and non-transformed shoots. A dose-response curve was constructed for wild-type Carrizo rootstock in liquid medium to evaluate the effects of kanamycin concentration on the number and the length of microshoots. Kanamycin at 200 mg/l was chosen as the optimal concentration for selection of transgenic mature citrus shoots in bioreactors. At this dose, most non-transgenic microshoots turned yellow and their lengths and numbers were significantly reduced in comparison to the no kanamycin controls. Selection of transgenic shoots in bioreactors was tested after Agrobacterium transformations of mature Carrizo and Valencia using three different binary vectors containing nptII as the selectable marker. Shoots developed on semi-solid medium and were transferred to temporary immersion bioreactors containing liquid MS medium with 200 mg/l kanamycin. After two weeks of culture in bioreactors, 21 dark green shoots were visually selected on the basis of color from a total of 6882 microshoots, and 17 of them (81%) were confirmed as transgenic with either the GUS histochemical assay, GFP fluorescence or PCR. Yellow shoots (5675) to be discarded from pTLAB21 and pCAMBIA2301 transformations were also tested for GUS or GFP expression and only one (0.01%) was positive. Kanamycin selection of mature transgenic shoots in temporary immersion bioreactors permitted transgenics to be visually distinguished on the basis of color, and reduced labor and consumable costs for PCR screening, particularly when reporter genes were not used.





Similar content being viewed by others
Abbreviations
- TIB:
-
Temporary immersion bioreactor
- GUS:
-
β-glucuronidase
- GFP:
-
Green fluorescent protein
- nptII:
-
Neomycin phosphotransferase II
- MS:
-
Murashige and Skoog
- Cv.:
-
Cultivar
- HLB:
-
Huanglongbing
- Nos:
-
Nopaline synthase
- 35S:
-
Cauliflower mosaic virus 35S promoter
- AtNPR1 :
-
Arabidopsis thaliana nonexpressor of pathogenesis-related gene
References
Albrecht U, Bowman KD (2011) Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco × Poncirus trifoliata L. Raf.) to Huanglongbing. HortScience 46(1):16–22
Albrecht U, Bowman KD (2012) Tolerance of trifoliate citrus rootstock hybrids to Candidatus Liberibacter asiaticus. Sci Hortic 147:71–80
Albrigo L, Syvertsen J (2015) Status of citrus fruit drop in relation to HLB citrus industry magazine. AgNet Media, Inc., Gainesville, 14–17
Ballester A, Cervera M, Peña L (2008) Evaluation of selection strategies alternative to nptII in genetic transformation of citrus plants. Plant Cell Rep 27(6):1005–1015
Browning H (2015) Integrating investments in HLB and other disease research citrus industry magazine. AgNet Media Inc., Gainesville, 15
Cervera M, Juarez J, Navarro L, Pena L (2005) Genetic transformation of mature citrus plants. In: Pena L (ed) Methods in molecular biology, 86. Humana Press, Totowa, pp 177–187
Chakrabarty D, Hahn E, Yoon Y, Paek K (2003) Micropropagation of apple rootstock M. 9 EMLA using bioreactor. J Hortic Sci Biotechnol 78(5):605–609
de Oliveira MLP, Febres VJ, Costa MGC, Moore GA, Otoni WC (2009) High-efficiency Agrobacterium-mediated transformation of citrus via sonication and vacuum infiltration. Plant Cell Rep 28(3):387–395
Domínguez A, Cervera M, Pérez RM, Romero J, Fagoaga C, Cubero J, López MM, Juárez JA, Navarro L, Peña L (2004) Characterisation of regenerants obtained under selective conditions after Agrobacterium-mediated transformation of citrus explants reveals production of silenced and chimeric plants at unexpected high frequencies. Mol Breed 14(2):171–183
Dutt M, Grosser J (2009) Evaluation of parameters affecting Agrobacterium-mediated transformation of citrus. Plant Cell Tissue Organ Cult (PCTOC 98(3):331–340
Escalona M, Lorenzo J, González B, Daquinta M, González J, Desjardins Y, Borroto C (1999) Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep 18(9):743–748
Febres V, Khalaf A, Moore GA, Fisher L (2011) Citrus transformation: challenges and prospects. INTECH Open Access, New York
Gatica-Arias A & Weber G (2013) Genetic transformation of hop (Humulus lupulus L. cv. Tettnanger) by particle bombardment and plant regeneration using a temporary immersion system. In Vitro Cell Dev Biol-Plant 49(6):656–664
Hanhineva KJ, Kärenlampi SO (2007) Production of transgenic strawberries by temporary immersion bioreactor system and verification by TAIL-PCR. BMC Biotechnol 7(1):1
Hidaka T, Omura M, Ugaki M, Tomiyama M, Kato A, Oshima M, Motoyoshi F (1990) Agrobacterium transformation and regeneration of citrus spp. from suspension cells. Jpn J Breed 40:119–207
Hu W, Li W, Xie S, Fagundez S, McAvoy R, Deng Z, Li Y (2016) Kn1 gene overexpression drastically improves genetic transformation efficiencies of citrus cultivars. Plant Cell Tissue Organ Cult (PCTOC 125(1):81–91
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6(13):3901
Kapaun JA, Cheng Z-M (1999) Aminoglycoside antibiotics inhibit shoot regeneration from siberian elm leaf explants. HortScience 34(4):727–729
Latawa J, Shukla MR & Saxena PK (2015) An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 94(1):1–8
Mallón R, Covelo P, Vieitez AM (2012) Improving secondary embryogenesis in Quercus robur: application of temporary immersion for mass propagation. Trees 26(3):731–741
Mallón R, Vieitez AM, Vidal N (2013) High-efficiency Agrobacterium-mediated transformation in Quercus robur: selection by use of a temporary immersion system and assessment by quantitative PCR. Plant Cell Tissue Organ Cult (PCTOC 114(2):171–185
Marchev A, Georgiev V, Ivanov I, Badjakov I, Pavlov A (2011) Two-phase temporary immersion system for Agrobacterium rhizogenes genetic transformation of sage (Salvia tomentosa Mill.). Biotechnol Lett 33(9):1873–1878
Moore G, Jacono C, Neidigh J, Lawrence S, Cline K (1992) Agrobacterium-mediated transformation of citrus stem segments and regeneration of transgenic plants. Plant Cell Rep 11(5–6):238–242
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Orbović V, Pasquali G & Grosser J (2007) A GFP-containing binary vector for Agrobacterium tumefaciens-mediated plant transformation. Paper presented at the ISHS International Symposium on Biotechnology of Temperate Fruit Crops and Tropical Species Daytona Beach, FL March 31, 2007
Peng A, Xu L, He Y, Lei T, Yao L, Chen S & Zou X (2015) Efficient production of marker-free transgenic ‘Tarocco’blood orange (Ci <background-color:#FFFFFF;i>trus sinensisOsbeck) with enhanced resistance to citrus canker using a Cre/loxP site-recombination system. Plant Cell Tissue Organ Cult (PCTOC) 123 (1):1–13
Wu H, Acanda Y, Shankar A, Peeples M, Hubbard C, Orbović V, Zale J (2015) Genetic transformation of commercially important mature citrus scions. Crop Sci 55:2786–2797
Zhang X, Francis MI, Dawson WO, Graham JH, Orbović V, Triplett EW, Mou Z (2010) Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. Eur J Plant Pathol 128(1):91–100
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acanda, Y., Canton, M., Wu, H. et al. Kanamycin selection in temporary immersion bioreactors allows visual selection of transgenic citrus shoots. Plant Cell Tiss Organ Cult 129, 351–357 (2017). https://doi.org/10.1007/s11240-017-1182-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1182-y