Abstract
This study is the first description of a procedure for shoot organogenesis from calli derived from various seedling explants. The frequency of shoot induction and number of shoots per explant were measured on Murashige and Skoog (MS) medium with 0.2–3.0 mg L−1 6-benzylaminopurine (BAP) alone or combined with auxin [0.1 mg L−1 indole-3-acetic acid (IAA) or 1-naphthalenacetic acid]. The highest organogenic response where 90 % of explants formed a regenerative callus with a mean number of nine shoots was achieved after 6 weeks of culture when hypocotyl explants and 1.0 mg L−1 BAP was used together with 0.1 mg L−1 IAA. The shoots regenerated from hypocotyl-derived calli were rooted for 6 weeks on MS medium lacking growth regulators or containing auxin (IAA or indole-3-butyric acid). The plantlets were successfully acclimatized in a greenhouse. Micropropagated plants subjected to random amplified polymorphic DNA and inter simple sequence repeat (ISSR) marker based profiling revealed a uniform banding pattern identical with that of donor plants. The iridoid and phenylethanoid glycoside content of the organogenic callus culture and in vitro-raised plants was determined using UHPLC. In the tested samples catalpol, aucubin, harpagid, harpagoside, verbascoside, isoverbascoside and traces of loganin and catalposide were identified. The leaves produced the highest levels of catalpol (43–45 mg g−1 DW). Organogenic callus and 6-week-old in vitro grown plantlets were characterized by the highest accumulation of aucubin, verbascoside and isoverbascoside. The work also demonstrated the regenerative ability of hypocotyl-derived calli and production of bioactive metabolites to be stable for 4 years of culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rehmannia glutinosa Libosch., a member of Orobanchaceae family, naturally occurs in China, Japan and Korea. Roots of the plant (Rehmanniae radix) have been listed in the Chinese Pharmacopoeia (2000) and have been widely used in Traditional Chinese Medicine as anti-senescence, hypoglycemic and anti-inflammatory agents (Zhang et al. 2008). The pharmacological properties of the plant roots have been attributed mainly to the presence of iridoid glycosides such as catalpol, aucubin, leonuride and melittoside (Zhang et al. 2008). The iridoids have been shown to have a range of biological activities. For example, catalpol possesses hypoglycemic and diuretic activities (Zhang et al. 2008), increases brain angiogenesis (Zhu et al. 2010), possesses extensive ischemic neural protection (Li et al. 2005) and attenuates apoptosis in the ischemic brain (Li et al. 2006). Another important class of natural products detected in the roots of R. glutinosa are phenylethanoid glycosides. Verbascoside (known as acteoside, kusaginin or orobanchin) exhibits a wide spectrum of biological activities, including antileukemic and cytotoxic activity against a murine cell line (Pettit et al. 1990). It demonstrates anti-inflammatory activity through inhibition of cyclooxygenase-2 (COX-2) expression and inhibition of complement activity in human serum (Gyurkovska et al. 2011), and also possesses diuretic (Herbert et al. 1991) and antibacterial properties (Pennacchio 2005). In turn, isoverbascoside has been found to demonstrate antibacterial and anti-inflammatory activities (Shikanga et al. 2010). Only one study describes the presence of iridoids and phenylethanoids in aerial parts of R. glutinosa (Albach et al. 2007): it notes that ethanolic extracts from dried R. glutinosa leaves contained catalpol, ajugol, 8-epiloganic acid, verbascoside and traces of rehmannoside A. As R. glutinosa has been overexploited for these pharmacologically active compounds, it has become endangered in its natural habitat and an efficient tissue culture system needs to be established to conserve this important and rare medicinal plant. A few studies describe the regeneration of R. glutinosa from leaf mesophil protoplasts (Xu and Davey 1983), leaves (Matsumoto et al. 1989; Park et al. 2009), root segments (Jeong et al. 2002) and shoot tips (Shoyama et al. 1983). In addition, one of our earlier studies describes the development of an in vitro propagation protocol for R. glutinosa using axillary buds (Piątczak et al. 2014). There is currently a lack of information regarding the use of seedling explants for shoot regeneration of R. glutinosa. The aim of the present study was to use aseptically grown seedlings of R. glutinosa to produce calli with the potential for shoot organogenesis and subsequent regeneration of the plant species accompanied by an analysis of the genetic status of the regenerants. So far, none of the developed R. glutinosa micropropagation protocols has assessed the genetic fidelity of micropropagated plants. The phytochemical part of the present study compares the production of iridoid and phenylethanoid glycosides in the shoot regenerating calli and micropropagated plants with that of seed-derived R. glutinosa plants.
Materials and methods
Plant material and explant preparation
Rehmannia glutinosa Libosch. seeds obtained from the Garden of Medicinal Plants of the Medical University in Wrocław (Poland) collected in 2006 were used in the study. The seeds were surface-sterilized and germinated as it was described earlier (Piątczak et al. 2014). As explants, hypocotyls, root segments and cotyledons (3–5 mm long) excised from 30-day-old aseptically growing seedlings were used.
Micropropagation of R. glutinosa through indirect organogenesis a adventitious shoots regenerated from hypocotyl-derived callus on agar MS medium supplemented with 1.0 mg L−1 BAP and 0.1 mg L−1 IAA after 4 weeks of culture, b a plantlet after 6 weeks of growth on agar MS medium with IAA (0.1 mg L−1), c in vitro-derived plant after 10 weeks of growth in the pot and d after 6 months of growth in the field. Bars 1 cm
Callus induction and shoot regeneration
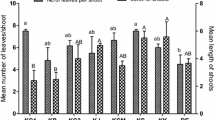
For initiation of organogenic callus hypocotyls, cotyledon and root segments excised from R. glutinosa seedlings were placed on MS (Murashige and Skoog 1962) 0.7 % agar (Sigma-Aldrich) media without PGRs (control) or supplemented with BAP (0.2, 0.5, 1.0 or 3.0 mg L−1) alone or with one of auxin: NAA or IAA at concentration 0.1 mg L−1. The pH of all media was adjusted to 5.6–5.8 by 0.1 N NaOH. For all experiments growth regulators were added prior to autoclaving. All media were autoclaved at 0.1 MPa, 121 °C, for 17 min. The cultures were kept in the growth chamber at 26 ± 2 °C under 16 h light/8 h dark photoperiod (cool-white fluorescent light; PPFD 40 μmol m−2 s−1). These incubation conditions were maintained throughout all experiments described in the study. After 6 weeks of culture the percentage of calli forming adventitious buds and/or shoots (regeneration frequency) as well as the average number of shoots produced per callus were recorded. Data were presented in Table 1. Calli derived from hypocotyls growing on MS medium with IAA (0.1 mg L−1) and BAP (1.0 mg L−1) (Fig. 1a) were selected for further experiments (shoot proliferation, rooting stage and phytochemical analysis). A small pieces of callus tissue (about 200–300 mg of fresh weight) with single adventitious bud were transferred into glass tubes with fresh medium. The calli were maintained on this medium for over 4 years (48 passages) with subculturing at 4-week intervals. Shoot regeneration capacity (i.e. the average number of shoots per callus) and the average length (mm) of the regenerated shoots were determined after 6, 12, 18, 24, 36 and 48 months (Fig. 2). Three replicates of ten calli were used for each culture age.
Changes in average numbers of adventitious shoots and average shoot length (mm) regenerated from hypocotyl-derived callus of R. glutinosa during long-term of in vitro culture on MS medium supplemented with BAP (1.0 mg L−1) and IAA (0.1 mg L−1) 4 weeks after transplanting into fresh medium. The values are the mean ± SE. The means followed by the same letter for the same series (number of shoots and their length) do not differ statistica at p ≤ 0.05 according to the Kruskal–Wallis test
Shoot rooting
Four-week-old shoots were excised from 4-year-old hypocotyl-derived calli and transferred into glass tubes containing MS medium without PGRs or supplemented with auxin (IAA or IBA) at concentration 0.1 mg L−1. After 6 weeks of culture the percentage of shoots forming roots, the average number of roots per shoot and their average length (mm) were recorded.
Plant acclimatization
Six-week-old rooted shoots were removed from the culture vessels and transferred into pots as it was described earlier (Piątczak et al. 2014). The survival rate was recorded after 10 weeks of growth in pots in a greenhouse. Greenhouse-plants were transferred into the field when they were approximately 12-week-old. Simultaneously, seed-derived plants were planted for comparison. Since no seeds germinated in the soil, they were germinated aseptically under in vitro conditions (MS medium without PGRs). Well developed 30-day-old seedlings were directly transferred into pots with soil. The seed-derived plants grew in greenhouse for 12 weeks and after that time they were transferred into the field.
DNA extraction
Genomic DNA was isolated from fresh leaves derived from fifteen 6-week-old plantlets obtained through indirect organogenesis from hypocotyl-derived callus from 48th subculture (4 years in in vitro culture) maintained on MS medium with IAA (0.1 mg L−1) and BAP (1.0 mg L−1) using a commercial DNA-extraction kit (NucleoSpin® Plant Core Kit Macherey-Nagel GmbH & Co. KG, Germany) according to the manufacturer’s protocol. The leaves from donor plants (seed-derived plants) were also used for comparison. For extraction, the leaf samples (about 200 mg) were powdered in liquid nitrogen and stored at −80 °C until used. Three replicate DNA extractions from leaves were used to assess the consistency of the band profiles. The quantity and quality of DNA samples were estimated by comparing band intensities on 1.5 % agarose gel stained with ethidium bromide (Sigma, Munich, Germany).
DNA amplification
Random amplified polymorphic DNA (RAPD) amplification was carried out in 25 μL reaction volume containing 2.5 μg DNA (2 μL), 2.5 μL of 10× PCR buffer (100 mM Tris–HCl, pH 8.3, 500 mM KCl, 11 mM MgCl2, 0.1 % gelatin), 1.5 mM MgCl2, 0.75 μL of 100 μM dNTP, 1,5 μL of RAPD primer, 0.75 µL of TaqNova DNA polymerase (DNA Gdańsk) and MilliQ water to make up the volume. RAPD amplification was performed with random decamer primers obtained from Blirt S.A. (Gdańsk, Poland). Five arbitrary RAPD primers were tested for PCR amplification. The PCR was performed at an initial denaturation at 95 °C for 5 min followed by 40 cycles of 1 min denaturation at 95 °C, 45 s annealing at 37 °C (and next depending on the primer, Table 2) and 2 min extension at 72 °C with a final extension of 72 °C for 7 min using a thermal cycler (BioRad, UK).
In case of Inter-simple sequence repeat (ISSR) primers, optimal annealing temperature was found to vary according to base composition of the primers. Eight ISSR primers (UBC primer, DNA Gdańsk, Poland) were used in the study. Amplification was carried out in 25 μL reaction volume containing 2.5 µg genomic DNA (2 μL) as template, 1.5 μL MgCl2, 0.75 μL of 100 μM dNTP, 3.5 μL of 10× PCR buffer (Taq buffer B devoid of MgCl2), 2.0 μL of ISSR primer (10 pM), 0.75 μL of TaqNova DNA polymerase (DNA Gdańsk) and MilliQ water to make up the volume. PCR amplifications were performed with initial denaturation at 95 °C for 5 min followed by 35 cycles of 30 s denaturation at 94 °C, 1 min at the annealing temperature (depending on the primer, Table 3), 2 min extension at 72 °C with a final extension of 72 °C for 10 min.
RAPD and ISSR amplifications were repeated three times and only the reproducible PCR products were scored. The amplification RAPD-PCR and ISSR-PCR products were resolved by electrophoresis on 1.5 % agarose gel (Bioline, UK), stained with ethidium bromide, measured with a 100 bp and 3 kb ladder (Fermentas, Lithuania) as the band size standard and photographed using DNR Bio-Imaging System MiniBIS Pro (Israel). The results were presented in Tables 2, 3 and Fig. 3.
Phytochemical analysis
UHPLC method
Four-week-old hypocotyl-derived caulogenic callus (6th–8th, 23rd–25th and 47th–49th subcultures) cultured on MS agar medium supplemented with BAP (1.0 mg L−1) and IAA (0.1 mg L−1), 6-week-old plantlets grown on agar MS medium supplemented with IAA (0.1 mg L−1), leaves and roots of 6-month-old in vitro regenerated plants were used for the experiments. For comparison, leaves and roots from 6-month-old seed-derived flowering plants were also used. The materials were lyophilized and powdered. The procedure of methanolic extract preparation was described in our earlier study (Piątczak et al. 2012). UHPLC analysis was used to determine iridoid (catalpol, aucubin, loganin, harpagide, harpagoside, catalposide) and phenylethanoid glycoside (verbascoside, isoverbascoside) contents. The analyses were carried out on Agilent Technologies 1290 Infinity apparatus equipped with diode array detector (DAD), a binary solvent delivery pump, vacuum degasser, an autosampler and thermostated column compartment. The separation was performed on a Zorbax Eclipse Plus C18 column (100 × 3.1 mm id; 1.8 μm Agilent Technologies) at 27 °C. The mobile phase consisted of 0.1 % formic acid in acetonitrile (v/v) (solvent A) and 0.1 % formic acid (v/v) in water (solvent B). A gradient programme was applied as follows: 0–1 min 1 % A; 1–4 min 1–15 % A; 4–6 min 15–21 % A; 6–9 min 21 % A; 9–11.5 min 21–90 % A; 11.5–15 min 90 % A. The column was equilibrated with 99 % A for 2 min between injections. The flow rate was 0.4 mL min−1. The injection volume was 0.3 μL. The detection wavelength was set at 204 nm (aucubin, catalpol and harpagide), 237 nm (loganin), 265 nm (catalposide), 280 nm (harpagoside) and 320 nm (acteoside and isoacteoside). The identification of determined compounds was made by comparing their UV spectra and the retention times of the peaks with those of the reference compounds purchased from PHYTOPLAN (Diehm & Neuberger GmbH, Heidelberg, Germany) and Roth (Karlsruhe, Germany), and by spiking the sample with standard solutions. The purity of the peaks was determined using the “peak purity” option in ChemStation 3D software to make sure that each determined peak contained only one compound. The compound concentrations were estimated by the interpolation of the peak areas with calibration curves constructed for a standard analytes. The contents of all analytes in the samples were expressed as mg of the compound per gram of dry weight (mg g−1 DW). The results are presented in Tables 4 and 5.
Statistical analysis
Shoot regeneration experiments were repeated three times in three subsequent treatments; ten explants per one treatment were used per each growth regulator concentration and each type of explant. Rooting experiments were also repeated three times. Total of 30 shoots were used per each rooting medium variant: MS medium without PGRs or supplemented with IAA or IBA (0.1 mg L−1). For plantlet acclimatization experiments, total 90 plantlets were taken (30 plantlets per each rooting medium variant). All analyses evaluating the iridoid and phenylethanoid glycoside contents were repeated three times. The results were expressed as mean values ± SE. Data were analyzed by Kruskal–Wallis test at the 0.05 significance level using STATISTICA 10 (STATSoft) Software.
Results and discussion
Shoot organogenesis from callus culture
Rehmannia glutinosa seedling explants (hypocotyls, cotyledon and root segments) were incubated for 6 weeks on MS basal medium with various levels of BAP (0.2, 0.5, 1.0 or 3.0 mg L−1) alone or in combination with IAA (0.1 mg L−1) or NAA (0.1 mg L−1) to encourage regeneration of adventitious shoots through indirect organogenesis. The seedling explants cultured on MS medium lacking plant growth regulators or containing only BAP at the lowest concentration tested (0.2 mg L−1) failed to produce callus, became brown and died. However, on the other media, friable and yellow–green calli appeared from the cut surface of the explants after 2–3 weeks of culture. A few days later, the callus tissue started to form dark green nodular structures showing meristematic centers which developed into buds and/or shoots during 6 weeks of culture. The explants responded positively by producing shoot regenerating calli at various frequencies corresponding to type and concentration of plant growth regulators, and type of explant (Table 1). The most efficient regeneration of shoots occurred with hypocotyl explants (Fig. 1a), with as many as 90 % of the explants being capable of forming organogenic calli, depending on the growth regulator used. The number of shoots ranged between 1.0 ± 0 and 9.2 ± 0.5 per responding explant. In contrast, the cotyledon explants demonstrated shoot induction in 80 % of calli and the mean number of shoots per callus ranged from 1.0 ± 0 to 6.9 ± 0.3. Similarly, hypocotyls have been reported to be more regenerative than cotyledons in Lessertia frutescens (Shaik et al. 2011) or Sanguisorba minor (Babaoglu and Yorgancilar 2000). In the present study, the lowest shoot regeneration capacity was achieved on root explants, which were found to produce max. 4.0 ± 0.7 shoots at a frequency of 53 %. Root segments have been found to be associated with little or no organogenesis in other plant species such as Carthamus tinctorius (Nikam and Shitole 1999). The difference in shoot regeneration capacity between explants could be due to different levels of endogenous hormones (Rao and Honnale 2011).
Beside choice of explant, plant growth regulators play an important role in determining the efficiency of shoot regeneration. A combination of BAP and auxin (0.1 mg L−1 IAA or 0.1 mg L−1 NAA) was found to be more effective in R. glutinosa shoot regeneration than BAP used individually (Table 1). Of all tested concentrations of BAP (0.2, 0.5, 1.0 and 3.0 mg L−1), the best organogenic response was achieved by a combination of 1 mg L−1 BAP and 0.1 mg L−1 IAA. After 6 weeks of culture on the medium 90 % of hypocotyl explants developed organogenic calli with a mean number of 9.2 ± 0.5 shoots per culture. The values decreased to 67 % and 1.5 ± 0.7 shoots per culture when 1.0 mg L−1 BAP was used individually. Also, cotyledon explants cultured in the presence of 1 mg L−1 BAP alone formed a lower percentage of organogenic calli (43 %) and fewer (1.2 ± 0.3) shoots per callus than those cultivated with a combination of 1 mg L−1 BAP and 0.1 mg L−1 IAA: these values being 80 % and 6.9 ± 0.3, respectively (Table 1). In the case of root explants, no significant difference (p ≤ 0.05) was observed between media containing 1 mg L−1 BAP (56.7 %) and a combination of 1 mg L−1 BAP and IAA (53.3 %) with regard to the frequency of explants forming organogenic calli. However, a significant difference was obtained when the number of shoots per responding explants was taken into account: 4.0 ± 0.7 versus 1.4 ± 0.5 per culture (Table 1). Auxin and cytokinin are known to exert a synergistic action on shoot regeneration from callus tissue in several plant species, for example Dorem ammoniacum (Irvani et al. 2010). According to Nordström et al. (2004) auxin can regulate cytokinin biosynthesis and reduce its level in plants. Therefore, a good balance between the two types of growth regulators is required. The findings of the present study indicate the number of regenerated shoots to be highest in R. glutinosa seedling explants when the ratio of cytokinin to auxin was 10:1. Of the two auxins IAA (0.1 mg L−1) and NAA (0.1 mg L−1), IAA was found to be more effective than NAA for organogenic callus induction. For example, in the presence of 1.0 mg L−1 BAP 43 % of hypocotyls produced organogenic calli with an average of 5.7 ± 1.3 adventitious shoots per callus when combined with 0.1 mg L−1 NAA, while 90 % of hypocotyls regenerated an average 9.2 ± 0.5 shoots per culture when combined with 0.1 mg L−1 IAA (Table 1). The number of regenerated shoots obtained in the present study using hypocotyl explants and MS medium supplemented with 1.0 mg L−1 BAP and 0.1 mg L−1 IAA was higher than that obtained in previous study with leaf explants of R. glutinosa (Park et al. 2009). In the study, a maximum of 3.8 shoots per explant were obtained using MS medium supplemented with 1.0 mg L−1 TDZ and 0.1 mg L−1 NAA (Park et al. 2009).
After the successful induction of primary organogenic callus, hypocotyl-derived calli and MS medium supplemented with BAP (1.0 mg L−1) and IAA (0.1 mg L−1) were selected for shoot multiplication, rooting stage and phytochemical analyses. As shown in Fig. 2, no significant differences were found in the number or length of shoots regenerated from the calli between successive subcultures at 4-week intervals for about 4 years of culture.
Rooting and plantlet acclimatization
Individual shoots induced on MS medium with 1.0 mg L−1 BAP and 0.1 mg L−1 IAA were excised from hypocotyl-derived calli and transferred on MS medium, either without growth regulators, or with either 0.1 mg L−1 IAA or IBA. Root induction was observed after 2 weeks and complete root development after 6 weeks of culture. Although 60 % of shoots developed an average four roots/shoot when rooted on medium devoid of auxin, the addition of auxin increase both the frequency of rooting and root length. Optimal rooting was achieved when the shoots were cultured on MS medium with 0.1 mg L−1 IAA: 93 % shoots developed an average five roots/shoot with a mean length of 44 mm within 6 weeks (Fig. 1b). The values were slightly lower on the medium containing 0.1 mg L−1 IBA: 83 % of shoots produced five roots with a mean length of 37 mm. However, no significant differences (p ≤ 0.05) were found between media containing IBA and IAA.
Six-week-old R. glutinosa plantlets with well developed roots were transferred from rooting media (MS without auxin or containing 0.1 mg L−1 IAA or IBA) into pots with soil, sand and peat. They were cultivated in the growth chamber for 2 weeks and then transplanted into the greenhouse (Fig. 1c). Of the 90 plants transferred to soil 86 survived the acclimatization period (10 weeks) giving a survival rate of 95 %, regardless of the presence or type of auxin in the rooting medium. The survival rate of in vitro-derived plants of R. glutinosa was higher than for plants obtained from the leaf explants (73 %) described by Park et al. (2009).
Molecular analysis
It is known that callus tissues are easily exposed to somaclonal variations, especially during long-term culture (Larkin and Snowcroft 1981). RAPD and ISSR are the favored methods used to determine the genetic stability of regenerants and these procedures were used to assess the genetic profile of R. glutinosa plants regenerated from 4-year-old callus culture. The obtained band patterns were compared between randomly-selected in vitro regenerants and donor plants. For RAPD-PCR analysis, five of the ten RAPD primers tested (Table 2) were selected for DNA amplification as they were found to produce distinct reproducible scorable bands. All these five RAPD-PCR primers resulted in the amplification of monomorphic bands (Fig. 3a), producing a total of 31 bands and an average of 6.2 bands per primer. The number and size range of the amplified scorable bands for each RAPD primer is presented in Table 2. Each primer generated a unique set of amplification products that were monomorphic across all regenerants and similar to those of the donor plants (Fig. 3a).
However, the eight selected primers used for the ISSR-PCR analysis yielded 45 clear, distinct bands, with an average of 5.6 bands per primer. Each ISSR primer generated a unique set of amplification products with sizes ranging from 300 to 2,000 bp (Table 3; Fig. 3b). The number of bands amplified in each ISSR primer ranged from 4 to 9. The RAPD and ISSR analyses revealed that the in vitro-derived regenerants of R. glutinosa shared the same banding patterns as the donor plants, confirming that no major genetic variation occurred in the tested certain parts of the genome in plantlets regenerated from a 4-year-old callus. Similarly, no genetic differences were reported by Yuan et al. (2009) in callus regenerated plantlets of Saussurea involucrata after 2 years of culture. On the other hand, Kuznetsova et al. (2006) described that variability of among Pisum sativum plantlets increased with the length of culture. The genetic variation ranged from 0 to 5.6 % after 8 months of culture, which increased to 10 % after 10 years of culture. RAPD and ISSR markers have been used to assess the genetic stability of in vitro-derived plantlets of many other plant species (Ray et al. 2006; Kishor and Devi 2009; Aggarval et al. 2010; Bhatia et al. 2011; Mohanty et al. 2011).
Secondary metabolite production
The iridoid and phenylethanoid glycoside contents of the R. glutinosa shoot-differentiating calli (Fig. 1a), the 6-week-old in vitro grown plantlets (Fig. 1b) and the shoots and roots of 6-month-old in vitro propagated plants grown in the field (Fig. 1d) were evaluated. These levels were compared with those found in the shoots and roots of R. glutinosa plants derived from seeds and grown in the field under the same conditions as the in vitro derived plants. UHPLC analyses of the methanolic extracts revealed the presence of catalpol, aucubin, loganin, catalposide, harpagide and harpagoside as well as verbascoside and isoverbascoside in tested samples. The compounds were identified by comparison of their retention times, UV spectra and ion mass spectra with authentic standards, as described in our previous work (Piątczak et al. 2012). Additionally, in the present study, LC–ESI–MS was performed to confirm the identity of the peaks of harpagide and harpagoside. These iridoids were found in R. glutinosa for the first time. Negative ionization LC–ESI–MS was used to further elucidate the iridoid structure: the quasi-molecular ions [M–H]− and adducted ions [M+HCOO]− were found to be the most abundant ions both for harpagide and harpagoside, while harpagoside was also found to be associated with the fragment ions [M–H–harpagide]− and [M–H–cinnamic acid]−. The information from the mass spectra combined with the standard compounds enable an unambiguous identification of harpagide and harpagoside in the studied R. glutinosa plant material.
Production of iridoid glycosides
The quantitative analyses of methanolic extracts demonstrated that catalpol was the predominant iridoid of tissue culture and R. glutinosa plants tested. The highest level of the iridoid was found in the leaves of tissue culture-derived field-grown plants (45.2 mg g−1 DW). This level was comparable to that seen in the leaves of plants grown directly from seeds (43.7 mg g−1 DW) but 5–6 times higher than that of the roots of both in vitro (9.5 mg g−1 DW) and in vivo (7.4 mg g−1 DW) generated plants as well as the leaves of R. glutinosa analysed by Albach et al. (2007): 28 mg g−1 DW. The fact that the highest catalpol content was recorded in leaves suggest that they may be the main organ for accumulation or biosynthesis of the compound. Catalpol concentrations in the leaves of in vitro regenerated and seed-derived R. glutinosa were also greater than those seen in crude R. radix from various locations in China which ranged from 0.06 to 19 mg g−1 DW (Xu et al. 2012). Conversely, the lowest level of catalpol (1.4 mg g−1 DW) was found in in vitro plantlets of R. glutinosa grown for 6 weeks on MS medium supplemented with IAA (0.1 mg L−1) (Table 5). This level was 2.5 times lower than that seen in the organogenic callus (3.3–3.6 mg g−1 DW) whose shoots were used to establish in vitro plantlets. This observed difference in catalpol accumulation between micropropagated plants grown in vitro (6-week-old) and in the field (6-month-old) could be the result of different environmental conditions and the developmental stage of plants. In addition, the exogenous supply of PGRs used during different micropropagation stages can also influence the level of secondary metabolites (Aremu et al. 2012).
Aucubin production was also examined in the present study (Tables 4, 5). It was detected in all tested samples but at much lower concentrations than catalpol. The highest concentrations were identified in organogenic callus culture (0.8 mg g−1 DW), followed by 6-week-old in vitro grown plantlets (0.3 mg g−1 DW) and the lowest levels of aucubin were found in the leaves (0.03 mg g−1 DW) and roots (0.02–0.03 mg g−1 DW) of mature (6-month-old) plants of R. glutinosa, originating from calli or seeds. Xu et al. (2012) report similar aucubin concentrations in R. glutinosa roots ranging from 0 to 0.029 mg g−1 DW depending on the location of the plant. The higher aucubin levels found in organogenic callus and in vitro R. glutinosa plantlets may be caused by aucubin being formed at an earlier developmental stage than other iridoids, and it may serve as a precursor for their biosynthesis. This hypothesis is supported by the findings of Rønsted et al. (2000), who identified aucubin as an intermediate involved in the biosynthesis of iridoids, including catalpol.
Other iridoids–loganin and catalposide were detected in low or trace amounts in intact plants of R. glutinosa grown in vitro or in the field (Table 5), but were not found in shoot-regenerating calli of R. glutinosa. Our earlier study has shown that catalposide, a 6-O-ester of catalpol, is the main iridoid in hairy roots of R. glutinosa (Piątczak et al. 2012). In contrast, the transformed roots did not produce catalpol. Catalpol is known to be the direct precursor of catalposide (Jensen et al. 2005). The presence of a very low level of catalposide and a high level of catalpol may suggest that in the analyzed R. glutinosa specimens, catalpol is not converted into catalposide. Catalposide has not yet been found in R. glutinosa.
The findings of the present study are the first to reveal the presence of harpagide and harpagoside in the Rehmannia genus. However, the substances have previously been found in closely related plants from Scrophulariaceae family, for example Oreosolen wattii (Jensen et al. 2008). Harpagoside and harpagide have been shown to suppress lipopolysaccharide induced nitric oxide synthase and cyclooxygenase-2 (COX-2) expression through inhibition of nuclear factor κβ activation (Georgiev 2013; Zhang et al. 2011). Harpagide possesses antiprotozoal properties, mainly against Leishmannia donovani (Tandesmir et al. 2008). The quantitative analysis described in the present study did not show any differences in harpagoside yield or its distribution between the aerial and underground parts of in vitro and in vivo-derived plants of R. glutinosa grown in the field for 6 months: all produced 0.08–0.12 mg g−1 DW of harpagoside (Table 5). These concentrations were significantly higher than those found in in vitro grown plantlets (0.04 mg g−1 DW) and shoot-differentiating calli (about 0.03 mg g−1 DW). However, the calli accumulated the highest amount of harpagide, which is believed to act as a precursor of harpagoside (Georgiev et al. 2013). This amount is approximately 2.5 times higher than the found in the roots of R. glutinosa, independently of the origin of the plant: in vitro or from seeds. Harpagide was not identified in the leaves. According to Collin (2001), growth, tissue differentiation and development of the plant body determine the location and accumulation of secondary metabolites in plant cell and tissue culture.
Production of phenylethanoid glycosides
The verbascoside and isoverbascoside content of the analyzed samples is presented in Tables 4 and 5. The highest levels of both phenylethanoids were found in organogenic callus culture of R. glutinosa: 12.5 mg g−1 DW verbascoside and 3.2 mg g−1 DW isoverbascoside. These values are three and seven times higher for verbascoside and isoverbascoside, respectively than those seen in the leaves of mature (6-month-old) in vivo and in vitro-derived plants. It should be also noticed that in underground parts of the plants, the verbascoside content was half that of the leaves. No isoverbascoside was detected in the roots. It is possible that improvement of verbascoside and isoverbascoside biosynthesis in cultured callus tissues and in vitro- grown plantlets of R. glutinosa is a response to stress induced by in vitro conditions.
The production of secondary metabolites in organogenic callus during long-term cultivation
UHPLC analyses of R. glutinosa organogenic callus performed after 6, 24 and 48 months of cultivation on MS medium supplemented with 1.0 mg L−1 BAP and 0.1 mg L−1 IAA showed that the culture represents a stable system for the production of secondary metabolites. No significant difference was observed in the qualitative and quantitative profiles of iridoids and phenylethanoids in the culture over a period of at least 4 years (Table 4). The study reveals that verbascoside content in organogenic callus culture during the whole cultivation period (4 years) was 3–6 times higher than in leaves or roots of R. glutinosa plants. In many other plant species callus cultures were reported to be a good source of verbascoside. For example, Estrada-Zúñiga et al. (2009) found that callus culture of Buddleja cordata produced 86 mg g−1 DW of verbascoside, compared to 10 and 68 mg g−1 DW in the leaves and roots of the wild plants, respectively. Inagaki et al. (1991) reported the production of the compound in callus cultures cultivated on MS medium with different concentrations of an auxin—2,4-d in ten various plant species including of R. glutinosa. They observed that the level of verbascoside ranged from 0.63 % of dry weigh (Syringa reflexa callus) to 6.33 % of dry weight (Stachys sieboldii callus).
On the other hand, there are a few examples of callus cultures that accumulated iridoids. Zakirova and Malikova (2000) reported the production of small amounts of harpagide by callus tissues of Ajuga turkestanica and Nakazawa and Toda (1995) found aucubin (0.03 mg g−1 of fresh weight) in callus cultures of Eucommia ulmoides. However, iridoids were not detected in callus tissues of Scrophularia nodosa (Sesterhenn et al. 2007) and Tecoma sambucifolium (Pletsch et al. 1993), although the compounds were present in the whole plant species.
Conclusions
In this study, high efficiency shoot organogenesis and plant regeneration were achieved from hypocotyl-derived calli of R. glutinosa. At least 680 plantlets can be produced per organogenic callus over three successive passages. The plants were successfully grown in the field. The results of RAPD and ISSR demonstrate that the in vitro propagation protocol did not induce genetic changes in the regenerants. The relatively high level of catalpol in the leaves of the plants indicates that they can be used as potential sources of the iridoid instead of wild plants. Additionally, the presence of harpagide, harpagoside and verbascoside may improve the pharmacological properties of the extracts obtained from the leaves of R. glutinosa plants regenerated via the callus stage. It is also important that the R. glutinosa callus maintained the ability to regenerate shoots and produce bioactive iridoid and phenylethanoid glycosides during long-term cultivation, making it suitable for biotechnological application.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- 2,4-D:
-
Dichloroacetic acid
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- ISSR:
-
Inter simple sequence repeat
- MS:
-
Murashige and Skoog medium (Murashige and Skoog 1962)
- NAA:
-
1-Naphtalenacetic acid
- PGRs:
-
Plant growth regulators
- RAPD:
-
Random amplified polymorphic DNA
- SE:
-
Standard error
References
Aggarval D, Kumar A, Reddy MS (2010) Shoot organogenesis in elite clones of Eucalyptus tereticornis. Plant Cell Tissue Organ Cult 102:45–52. doi:10.1007/s11240-010-9303-y
Albach DC, Li HQ, Zhao N, Jensen SR (2007) Molecular systematics and phytochemistry of Rehmannia (Scrophulariaceae). Biochem Syst Ecol 35:293–300. doi:10.1016/j.bse.2006.11.003
Aremu AO, Bairu MW, Szűčová L, Doležal K, Finnie JF, van Staden J (2012) Assessment of the role of meta-topolins on in vitro produced phenolics and acclimatization competence of micropropagated ‘Williams’ banana. J Plant Physiol 170:1303–1308. doi:10.1007/s11738-012-1027-6
Babaoglu M, Yorgancilar M (2000) TDZ-specific plant regeneration in salad burnet. Plant Cell Tissue Organ Cult 440:31–34
Bhatia R, Singh KP, Sharma TR, Jhang T (2011) Evaluation of the genetic fidelity of in vitro-propagated gerbera (Gerbera jamesonii Bolus) using DNA-based markers. Plant Cell Tissue Organ Cult 104:131–135. doi:10.1007/s11240-010-9806-5
Collin HA (2001) Secondary product formation in plant tissue cultures. Plant Growth Regul 34:119–134
Estrada- Zúñiga ME, Cruz-Sosa F, Rodrĩguez-Monroy M, Verde-Calvo JR, Vernon-Carter EJ (2009) Phenylpropanoid production in callus and cell suspension cultures of Buddleja cordata Kunth. Plant Cell Tissue Organ Cult 97:39–47. doi:10.1007/s11240-009-9496-z
Georgiev MI, Ivanovska N, Alipieva K, Dimitrova P, Verpoorte R (2013) Harpagoside: from Kalahari Desert to pharmacy shelf. Phytochemistry 92:8–15. doi:10.1016/j.phytochem.2013.04.009
Gyurkovska V, Alipieva K, Maciuk A, Dimitrova P, Ivanovska N, Haas C, Bley T, Georgiev M (2011) Anti-inflammatory activity of devil’s claw in vitro systems and their active constituents. Food Chem 125:171–178. doi:10.1016/j.foodchem.2010.08.056
Herbert JM, Mafrand JP, Toaubi K, Augereau JM, Fouraste I, Gleye J (1991) Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J Nat Prod 54:1595–1600. doi:10.1021/np50078a016
Inagaki N, Nishimura H, Okada M, Mitsuhashi H (1991) Verbascoside production by plant cell cultures. Plant Cell Rep 9:484–487
Irvani N, Solouki M, Omidi M, Zare AR, Shahnazi S (2010) Callus induction and plant regeneration in Dorem ammoniacum D., an endangered medicinal plant. Plant Cell Tissue Organ Cult 100:293–299. doi:10.1007/s11240-009-9650-7
Jensen SR, Albach DC, Ohno T, Grayer RJ (2005) Veronica: iridoids and cornoside as chemosystematic markers. Biochem Syst Ecol 33:1031–1047. doi:10.1016/j.bse.2005.03.001
Jensen SR, Li HQ, Albach DC, Gotfredsen CH (2008) Phytochemistry and molecular systematics of Triaenophora rupestris and Oreosolen wattii (Scrophulariaceae). Phytochemistry 69:2162–2166. doi:10.1016/j.phytochem.2008.05.010
Jeong JH, Yu KW, Chakrabarty D, Kim SJ, Kee Y, Paek KY (2002) In vitro regeneration and plantlet formation from adventitious roots of Rehmannia glutinosa Liboschits. Propag Ornam Plants 2:19–23
Kishor R, Devi HS (2009) Induction of multiple shoots in a monopodial orchid hybrid (Aerides vandarum Reichb.f × Vanda stangeana Reichb.f) using thidiazuron and analysis of their genetic stability. Plant Cell Tissue Organ Cult 97:121–129. doi:10.1007/s11240-009-9506-1
Kuznetsova OI, Ash OA, Gostimsky SA (2006) The effect of the duration of callus culture on the accumulation of genetic alterations in pea Pisum sativum L. Russ J Genet 42:555–562. doi:10.1134/S1022795406050139
Larkin PJ, Snowcroft WR (1981) Somaclonal variation—a novel source of variability from cells cultures for plant improvement. Theor Appl Genet 60:197–214
Li DQ, Li Y, Liu Y, Bao YMHuB, An LJ (2005) Catalpol prevents the loss of CA1 hippocampal neurons and reduces working errors in gerbils after ischemia–reperfusion injury. Toxicon 46:845–851. doi:10.1016/j.toxicon.2004.09.007
Li DQ, Bao YM, Li Y, Wang CF, Liu Y, An LJ (2006) Catalpol modulates the expressions of Bcl-2 and Bax and attenuates apoptosis in gerbils after ischemic injury. Brain Res 1115:179–185. doi:10.1016/j.brainres.2006.07.063
Matsumoto M, Shoyama Y, Nishioka I, Irino N (1989) Constituents of regenerated shoot and cultured root tissue of Rehmannia glutinosa. Phytochemistry 28:2331–2332
Mohanty S, Parada R, Singh S, Joshi RK, Subudhi E, Nabak S (2011) Biochemical and molecular profiling of micropropagated and conventionally grown Kaempferia galangal. Plant Cell Tissue Organ Cult 106:39–46. doi:10.1007/s11240-010-9891-5
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco culture. Physiol Plant 15:473–497
Nakazawa Y, Toda Y (1995) Eucommia ulmoides Oliv. (Eucommiaceae): In-vitro culture and the production of iridoids, lignans, and other secondary metabolites. In: Bajaj APS (ed) Medicinal and aromatic plants, vol 8. Springer, Berlin, pp 215–331
Nikam TD, Shitole MG (1999) In vitro culture of Safflower L. cv. Bhima: initiation, growth optimization and organogenesis. Plant Cell Tissue Organ Cult 55:15–22
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Doleżał K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. PNAS 101:8039–8044. doi:10.1073/pnas.0402504101
Park SU, Kim YK, Lee SY (2009) Improved in vitro plant regeneration and micropropagation of Rehmannia glutinosa L. J Med Plants Res 3:31–34
Pennacchio M (2005) Traditional Australian aboriginal bush medicine. HerbalGram 65:38–44
Pettit GR, Numata A, Takemura T, Ode RH, Narula AS, Schmidt JM, Cragg GM, Pase CP (1990) Antineoplastic agents, 107. Isolation of acteoside and isoacteoside from Castilleja linariaefolia. J Nat Prod 53:456–458. doi:10.1021/np50068a026
Pharmacopoeia Commission of the People`s Republic of China (2000) The pharmacopoeia of the People`s Republic of China, vol 1. Chemical Industry Publishing House, Beijing, p 94
Piątczak E, Królicka A, Wielanek M, Wysokińska H (2012) Hairy root cultures of Rehmannia glutinosa and production of iridoid and phenylethanoid glycosides. Acta Physiol Plant 34:2215–2224. doi:10.1007/s11738-012-1022-y
Piątczak E, Grzegorczyk-Karolak I, Wysokińska H (2014) Micropropagation of Rehmannia glutinosa Libosch.: production of phenolics and flavonoids and evaluation of antioxidant activity. Acta Physiol Plant 36:1693–1702. doi:10.1007/s11738-014-1544-6
Pletsch M, Piacente S, Pizza C, Charlwood BV (1993) The accumulation of phenylpropanoid glycosides in tissue cultures of Tecoma sambucifolium. Phytochemistry 34:161–165
Rao S, Honnale HN (2011) Callus induction and organogenesis in Sesamum indicum L. cv. E 8. Curr Trends Biotechnol Pharm 5:1462–1468
Ray T, Dutta I, Saha P, Das S, Roy SC (2006) Genetic stability of three economically important micropropagated banana (Musa spp.) cultivars of lower Indo-Gangetic plains, as assessed by RAPD and ISSR markers. Plant Cell Tissue Organ Cult 85:11–21. doi:10.1007/s11240-005-9044-4
Rønsted N, Gøbel E, Franzyk H, Jensen SR, Olsen CE (2000) Chemotaxonomy of Plantago iridoid glucosides and cafeoyl phenylethanoid glycosides. Phytochemistry 55:337–348
Sesterhenn K, Distl M, Wink M (2007) Occurrence of iridoid glycosides in in vitro cultures and intact plants of Scrophularia nodosa L. Plant Cell Rep 26:365–371. doi:10.1007/s00299-006-0233-3
Shaik S, Singh N, Nicholas A (2011) Cytokinin-induced organogenesis in Lessertia (Sutherlandia) frutescens L. using hypocotyl and cotyledon explants affects yields of L-canavanine in shoots. Plant Cell Tissue Organ Cult 105:439–446. doi:10.1007/s11240-010-9885-3
Shikanga EA, Combrinck S, Regnier T (2010) South African Lippia herbal infusions: total phenolic content, antioxidant and antibacterial activities. S Afr J Bot 76:567–571. doi:10.1016/j.sajb.2010.04.010
Shoyama Y, Nagano M, Nishioka I (1983) Clonal multiplication of Rehmannia glutinosa. Planta Med 48:124–128
Tandesmir D, Brun R, Franzblan SG, Sezgin Y, Calis I (2008) Evaluation of antiprotozoal and antimycobacterial activities of the resin glycosides and the other metabolites of Scrophularia cryptophila. Phytomedicine 15:209–215. doi:10.1016/j.phymed.2007.07.032
Xu YH, Davey MR (1983) Shoot regeneration from mesophyll protoplasts and leaf explants of Rehmannia glutinosa. Plant Cell Rep 2:55–57
Xu J, Wua J, Zhu LY, Shen H, Xu JD, Jensen SR, Jia XB, Zhang QW, Li SL (2012) Simultaneous determination of iridoid glycosides, phenethylalcohol glycosides and furfural derivatives in Rehmanniae radix by high performance liquid chromatography coupled with triple-quadrupole mass spectrometry. Food Chem 135:2275–2286. doi:10.1016/j.foodchem.2012.07.006
Yuan XF, Dai ZH, Wang XD, Zhao B (2009) Assessment of genetic stability in tissue-cultured products and seedlings of Saussarea involucrata by RAPD and ISSR markers. Biotechnol Lett 31:1279–1287. doi:10.1007/s10529-009-9984-6
Zakirova RP, Malikova MK (2000) Effect of N-nitroso-N-methylurea on the biosynthetic activity of Ajuga turkestanica callus tissue. Chem Nat Compd 36:384–386
Zhang RX, Li MX, Jia ZP (2008) Rehmannia glutinosa: review of botany, chemistry and pharmacology. J Ethnopharmacol 117:199–214. doi:10.1016/j.jep.2008.02.018
Zhang L, Feng L, Jia Q, Xu J, Wang R, Wang Z, Wu Y, Li Y (2011) Effects of β-glucuronidase hydrolyzed products of harpagide and harpagoside on cyclooxygenase-2 (COX-2) in vitro. Bioorg Med Chem. 19:4882–4886. doi:10.1016/j.bmc.2011.06.06
Zhu HF, Wan D, Luo Y, Zhou JL, Chen L, Xu XY (2010) Catalpol increases brain angiogenesis and up-regulates VEGF and EPO in the rat after permanent middle cerebral artery occlusion. Int J Biol Sci 6:443–453
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Piątczak, E., Kuźma, Ł., Sitarek, P. et al. Shoot organogenesis, molecular analysis and secondary metabolite production of micropropagated Rehmannia glutinosa Libosch.. Plant Cell Tiss Organ Cult 120, 539–549 (2015). https://doi.org/10.1007/s11240-014-0620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0620-3