Abstract
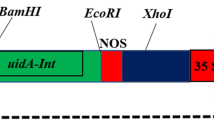
An improved bacterial preculture protocol for Agrobacterium-mediated genetic transformation was developed for an economic tomato cultivar (Solanum lycopersicum L. cv. Zhongshu No. 4). Frequencies of transient gene expression and stable transformation were influenced by the density of Agrobacterium preculture and not the density of Agrobacterium used for infection. The improved protocol presented in this study depends on the use of an overnight-grown Agrobacterium preculture density of OD600 nm = 1.0, diluted 1/10th with Luria-Bertani (LB) liquid medium, and grown for an additional 4 h. Cultures are collected and resuspended in a liquid cocultivation medium-I, adjusted to OD600 nm = 0.1. Using this modified Agrobacterium preparation, transient β-glucuronidase expression was higher than 90%, and transformation efficiency reached 44.7%. This improved transformation is simple, repeatable, does not require a feeder layer, and most notably, the transformation frequency is stable and highly efficient.







Similar content being viewed by others
References
Abel S, Ticconi CA, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115:1–8. doi:10.1034/j.1399-3054.2002.1150101.x
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424. doi:10.1007/s00299-007-0474-9
Chyi YS, Phillips GC (1987) High efficiency Agrobacterium-mediated transformation of Lycopersicon based on conditions favorable for regeneration. Plant Cell Rep 6:105–108. doi:10.1007/BF00276664
Cortina C, Culiáňez-Macià FA (2004) Tomato transformation and transgenic plant production. Plant Cell Tissue Organ Cult 76:269–275. doi:10.1023/B:TICU.0000009249.14051.77
Dan Y, Yan H, Munyikwa T, Dong J, Zhang Y, Armstrong CL (2006) MicroTom—a high-throughput model transformation system for functional genomics. Plant Cell Rep 25:432–441. doi:10.1007/s00299-005-0084-3
Dillen W, DeClercq J, Kapila J, Zambre M, VanMontagu M, Angenon G (1997) The effect of temperature on Agrobacterium tumefaciens-mediated gene transfer to plants. Plant J 12:1459–1463. doi:10.1046/j.1365-313x.1997.12061459.x
Ellul P, Garcia-Sogo B, Pineda B, Ríos G, Roig LA, Moreno V (2003) The ploidy level of transgenic plants in Agrobacterium-mediated transformation of tomato cotyledons (Lycopersicon esculentum L.) is genotype and procedure dependent. Theor Appl Genet 106:231–238. doi:10.1007/s00122-002-0928-y
Frary A, Earle ED (1996) An examination of factors affecting the efficiency of Agrobacterium-mediated transformation of tomato. Plant Cell Rep 16:235–240. doi:10.1007/BF01890875
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67:16–37. doi:10.1128/MMBR.67.1.16-37.2003
Hamza S, Chupeau Y (1993) Re-evaluation of conditions for plant regeneration and Agrobacterium-mediated transformation from tomato (Lycopersicon esculentum). J Exp Bot 44:1837–1845. doi:10.1093/jxb/44.12.1837
Hansen G, Das A, Chilton MD (1994) Constitutive expression of the virulence genes improves the efficiency of plant transformation by Agrobacterium. Proc Natl Acad Sci USA 91:7603–7607
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231. doi:10.1126/science.227.4691.1229
Hwang HH, Gelvin SB (2004) Plant proteins that interact with VirB2, the Agrobacterium tumefaciens pilin protein, mediate plant transformation. Plant Cell 16:3148–3167. doi:10.1105/tpc.104.026476
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Joubert P, Sangwan RS, EI Arabi Aouad M, Beaupère D, Sangwan-Norreel BS (1995) Influence of phenolic compounds on Agrobacterium vir gene induction and onion gene transfer. Phytochemistry 40:1623–1628. doi:10.1016/0031-9422(95)00539-J
Krasnyanski SF, Sandhu J, Domier LL, Buetow DE, Korban SS (2001) Effect of an enhanced CaMV 35S promoter and a fruit-specific promoter on uida gene expression in transgenic tomato plants. In Vitro Cell Dev Biol Plant 37:427–433. doi:10.1007/s11627-001-0075-1
Ling HQ, Kriseleit D, Ganal MW (1998) Effect of ticarcillin/potassium clavulanate on callus growth and shoot regeneration in Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum Mill.). Plant Cell Rep 17:843–847. doi:10.1007/s002990050495
Lipp João KH, Brown TA (1993) Enhanced transformation of tomato co-cultivated with Agrobacterium tumefaciens C58C1Rifr::pGSFRll61 in the presence of acetosyringone. Plant Cell Rep 12:422–425. doi:10.1007/BF00234705
McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R (1986) Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep 5:81–84. doi:10.1007/BF00269239
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ogaki M, Furuichi Y, Kuroda K, Chin DP, Ogawa Y, Mii M (2008) Importance of co-cultivation medium pH for successful Agrobacterium-mediated transformation of Lilium × formolongi. Plant Cell Rep 27:699–705. doi:10.1007/s00299-007-0481-x
Park SH, Morris JL, Park JE, Hirschi KD, Smith RH (2003) Efficient and genotype-independent Agrobacterium-mediated tomato transformation. J Plant Physiol 160:1253–1257. doi:10.1078/0176-1617-01103
Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA (2005) Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA 102:18830–18835. doi:10.1073/pnas.0509512102
Pozueta-Romero J, Houlné G, Cañas L, Schantz R, Chamarro J (2001) Enhanced regeneration of tomato and pepper seedling explants for Agrobacterium-mediated transformation. Plant Cell Tissue Organ Cult 67:173–180. doi:10.1023/A:1011997926381
Qiu D, Diretto G, Tavarza R, Giuliano G (2007) Improved protocol for Agrobacterium mediated transformation of tomato and production of transgenic plants containing carotenoid biosynthetic gene CsZCD. Sci Hortic 112:172–175. doi:10.1016/j.scienta.2006.12.015
Raghothama KG (2000) Phosphate transport and signaling. Curr Opin Plant Biol 3:182–187. doi:10.1016/S1369-5266(00)80063-1
Raj SK, Singh R, Pandey SK, Singh BP (2005) Agrobacterium-mediated tomato transformation and regeneration of transgenic lines expressing tomato leaf curl virus coat protein gene for resistance against TLCV infection. Curr Sci 88:1674–1679
Sun HJ, Uchii S, Watanabe S, Ezura H (2006) A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol 47:426–431. doi:10.1093/pcp/pci251
Tabaeizadeh Z, Agharbaoui Z, Harrak H, Poysa V (1999) Transgenic tomato plants expressing a Lycopersicon chilense chitinase gene demonstrate improved resistance to Verticillium dahliae race 2. Plant Cell Rep 19:197–202. doi:10.1007/s002990050733
Uranbey S, Sevimay CS, Kaya MD, İpek A, Sancak C, Başalma D, Er C, Őzcan S (2005) Influence of different co-cultivation temperatures, periods and media on Agrobacterium tumefaciens-mediated gene transfer. Biol Plant 49:53–57. doi:10.1007/s10535-005-3057-z
van Roekel JSC, Damm B, Melchers LS, Hoekema A (1993) Factors influencing transformation frequency of tomato (Lycopersicon esculentum). Plant Cell Rep 12:644–647. doi:10.1007/BF00232816
Vidya CSS, Manoharan M, Kumar CTR, Savithri HS, Sita GL (2000) Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum var. Pusa Ruby) with coat-protein gene of Physalis mottle tymovirus. J Plant Physiol 156:106–110
Wroblewski T, Tomczak A, Michelmore R (2005) Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J 3:259–273. doi:10.1111/j.1467-7652.2005.00123.x
Wu Y, Chen Y, Liang X, Wang X (2006) An experimental assessment of the factors influencing Agrobacterium-mediated transformation in tomato. Russ J Plant Physiol 53:252–256. doi:10.1134/S1021443706020166
Yuan ZC, Edlind MP, Liu P, Saenkham P, Banta LM, Wise AA, Ronzone E, Binns AN, Kerr K, Nester EW (2007) The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching gene in Agrobacterium. Proc Natl Acad Sci USA 104:11790–11795. doi:10.1073/pnas.0704866104
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. doi:10.1146/annurev.arplant.53.091401.143329
Acknowledgments
The authors thank Dr. Sumei Li from the Institute of Soil Science, Chinese Academy of Sciences, for valuable suggestions on transgenic techniques. The authors also thank the anonymous reviewers for their valuable comments and suggestions that had improved this manuscript greatly. This work was supported by the National Natural Science Foundation of China (40671100) and the National Basic Research Program of China (2007CB109303).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, N., Shen, W., Cao, Y. et al. Influence of bacterial density during preculture on Agrobacterium-mediated transformation of tomato. Plant Cell Tiss Organ Cult 98, 321–330 (2009). https://doi.org/10.1007/s11240-009-9566-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9566-2