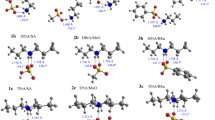
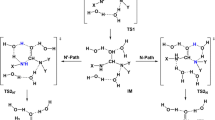
We have calculated the potential energy surface for arenesulfonylation reactions of a-amino acids and glycine hydrates. We have shown that all the reactions occur via a complicated route, with varying angle of attack by the nucleophile according to an S N2 mechanism. Hydration of glycine lowers the activation energy compared with the gas phase.



Similar content being viewed by others
References
V. A. Savelova and N. M. Oleinik, Mechanisms of Action for Organic Catalysts. Bifunctional and Intramolecular Catalysis [in Russian], Nauk. Dumka, Kiev (1990).
L. M. Litvinenko, V. A. Savelova, T. N. Solomoichenko, and V. G. Zaslavskii, Structure, Reactivity of Organic Compounds, and Reaction Mechanisms: Collected Scientific Papers [in Russian], Nauk. Dumka, Kiev (1980), pp. 3–68.
L. M. Litvinenko and N. M. Oleinik, Organic Catalysts and Homogeneous Catalysis [in Russian], Nauk. Dumka, Kiev (1981).
N. G. Shcheglova, T. P. Kustova, L. B. Kochetova, and N. V. Kalinina, Zh. Obshch. Khim., 79, No. 4, 631–633 (2009).
T. P. Kustova, N. G. Shcheglova, and N. V. Kalinina, Izv. Vuzov, Khimiya, Khim. Tekhnol., 51, No. 6, 26–30 (2008).
T. P. Kustova, N. G. Shcheglova, L. B. Kochetova, and N. V. Kalinina, Zh. Obshch. Khim., 80, No. 5, 802–805 (2010).
V. A. Savelova, “Kinetics and catalysis of nucleophilic substitution reactions in a series of sulfonic and carboxylic acid derivatives,” Author’s Abstract, Dissertation in competition for the academic degree of Doctor of Chemical Sciences, Donetsk (1986).
L. V. Kuritsyn, “Investigation of the effect of the nature of the solvent and the structure of the reagents on the acylation rate of aromatic amines,” Author’s Abstract, Dissertation in competition for the academic degree of Doctor of Chemical Sciences, Ivanovo (1975).
L. B. Kochetova and T. P. Kustova, Izv. Vuzov, Khimya Khim. Tekhnol., 52, No. 5, 12–15 (2009).
HyperChem Release 7.52 for Windows. Molecular Modeling System. Serial No. 12-750-1503700446, Hypercube, Inc., Gainesville (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 47, No. 1, pp. 56–60, January-February, 2011.
Rights and permissions
About this article
Cite this article
Kochetova, L.B., Kustova, T.P., Kalinina, N.V. et al. Quantum-chemical modeling of the mechanism for reaction of arenesulfonyl chlorides with α-amino acids. Theor Exp Chem 47, 61–66 (2011). https://doi.org/10.1007/s11237-011-9186-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-011-9186-x