Abstract
Context
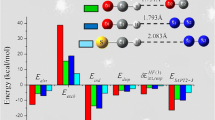
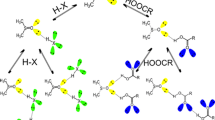
Proton transfer in acid–base systems is not well understood. Some acid–base reactions do not proceed to the extent that is expected from the difference in the pKa values between the base and acid in aqueous solutions, yet some do. In that regard, we have computationally studied the process of proton transfer from the acids of varying strength (benzenesulfonic acid (BSu), methansulfonic acid (MsO), and sulfuric acid (SA)) to the amines with different numbers of propyl substituents on the nitrogen atom (propylamine (PrA), dipropylamine (DPrA), and tripropylamine (TPrA)) upon complexation. Density functional theory calculations were used to thoroughly examine the energetic and structural aspects of the molecular complexes and/or ionic pairs resulting from the acid–base interaction. The potential energy curves along the proton transfer coordinate in these acid–amine systems were analyzed. The change in free energies accompanying the molecular complexes and ionic pair formations was calculated, and the relationship between the energy values and the ΔРА parameter (difference in proton affinity of the acid anion and amine) was established. The larger ΔРА values were found to be unfavorable for the formation of ionic pairs. Using structural, energy, QTAIM, and NBO analyses, we determined that the hydrogen bonds in the molecular complexes PrA-MsO and PrA-BSu are stronger than those in their corresponding ionic pairs. The ionic pairs with the TPrA cation possess the strongest hydrogen bonds of all the ionic pairs being studied, regardless of the anion. The results showed that hydrogen bonding interactions in the molecular complexes contribute significantly to the energies of the acid–base interaction, while in the ionic pairs, the most important energy contribution comes from Coulomb interactions, followed by hydrogen bonding and dispersion forces. The ionic pairs with propylammonium, dipropylammonium, and tripropylammonium cations have stronger ion–ion interactions than tetrapropylammonium (TetPrA)-containing ionic pairs with the same anions. This effect rises with the order of the cation: TetPrA → TPrA → DPrA → PrA, and the sequence of anions is SA → BSu → MsO. The results obtained here expand the concept of acid–base interaction and provide an alternative to experimental searches for suitable acids and bases to obtain new types of protic ionic liquids.
Methods
All quantum-chemical calculations were carried out by using the DFT/B3LYP-GD3/6-31++G(d,p) level as implemented in the Gaussian 09 software package. For the resulting structures, the electron density distribution was analyzed by the “atoms in molecules” (QTAIM) and the natural bond orbital (NBO) methods on the wave functions obtained at the same level of theory by AIMAll Version 10.05.04 and Gaussian NBO Version 3.1 programs, respectively.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Plechkova NV, Seddon KR (2008) Application of ionic liquids in the chemical industry. Chem Soc Rev 37:123–150. https://doi.org/10.1039/B006677J
Stoimenovski J, Dean PM, Izgorodina EI, MacFarlane DR (2012) Protic pharmaceuticallionic liquids and solids: aspects of protonic. Faraday Discuss 154:335–352. https://doi.org/10.1039/C1FD00071C
Liu H, Yu H (2019) Ionic liquids for electrochemical energy storage devices applications. J Mater Sci Technol 35:674–686. https://doi.org/10.1016/j.jmst.2018.10.007
Elwan HA, Mamlouk M, Scott K (2021) A review of proton exchange membranes based on protic ionic liquid/polymer blends for polymer electrolyte membrane fuel cells. J Power Sources 484:229197. https://doi.org/10.1016/j.jpowsour.2020.229197
Vázquez-Fernández I, Raghibi M, Bouzina A, Timperman L, Bigarré J, Anouti M (2021) Protic ionic liquids/poly(vinylidene fluoride) composite membranes for fuel cell application. J Energy Chem 53:197–207. https://doi.org/10.1016/j.jechem.2020.04.022
Sun X-L, Deng W-H, Chen H, Han H-L, Taylor JM, Wan C-Q, Xu G (2017) A metal-organic framework impregnated with a binary ionic liquid for safe proton conduction above 100°C. Chem Eur J 23:1248–1252. https://doi.org/10.1002/chem.201605215
Nuthakki B, Greaves TL, Krodkiewska I, Weerawardena A, Burgar MI, Mulder RJ, Drummond CJ (2007) Protic ionic liquids and ionicity. Aust J Chem 60:21–28. https://doi.org/10.1071/CH06363
Greaves TL, Drummond CJ (2008) Protic ionic liquids: properties and applications. Chem Rev 108:206–237. https://doi.org/10.1021/cr068040u
Cruz-Cabeza AJ (2012) Acid-base crystalline complexes and the pKa rule. CrystEngComm 14:6362–6365. https://doi.org/10.1039/c2ce26055g
Yoshizawa M, Xu W, Angell CA (2003) Ionic liquids by proton transfer: vapor pressure, conductivity, and the relevance of ΔpKa from aqueous solutions. J Am Chem Soc 125:15411–15419. https://doi.org/10.1021/ja035783d
Miran MS, Kinoshita H, Yasuda T, Susan MABH, Watanabe M (2011) Hydrogen bonds in protic ionic liquids and their correlation with physicochemical properties. Chem Commun 47:12676–12678. https://doi.org/10.1039/c1cc14817f
Miran MS, Kinoshita H, Yasuda T, Susan MABH, Watanabe M (2012) Physicochemical properties determined by ΔpKa for protic ionic liquids based on an organic super-strong base with various brønsted acids. Phys Chem Chem Phys 14:5178–5186. https://doi.org/10.1039/c2cp00007e
Stoimenovski J, Izgorodina EI, MacFarlane DR (2010) Ionicity and proton transfer in protic ionic liquids. Phys Chem Chem Phys 12:10341–10347. https://doi.org/10.1039/c0cp00239a
Reid JESJ, Bernardes CES, Agapito F, Martins F, Shimizu S, Minas da Piedade ME, Walker AJ (2017) Structure-property relationships in protic ionic liquids: a study of solvent-solvent and solvent-solute interactions. Phys Chem Chem Phys 19:28133–28138. https://doi.org/10.1039/c7cp05076c
Pant R, Kumar M, Venkatnathan A (2017) Quantum mechanical investigation of proton transport in imidazolium methanesulfonate ionic liquid. J Phys Chem C 121:7069–7080. https://doi.org/10.1021/acs.jpcc.6b11997
Nasrabadi AT, Gelb LD (2018) How proton transfer equilibria influence ionic liquid properties: molecular simulations of alkylammonium acetates. J Phys Chem B 122:5961–5971. https://doi.org/10.1021/acs.jpcb.8b01631
Ingenmey J, Gehrke S, Kirchner B (2018) How to harvest grotthuss diffusion in protic ionic liquid electrolyte systems. ChemSusChem 11:1900–1910. https://doi.org/10.1002/cssc.201800436
Davidowski SK, Thompson F, Huang W, Hasani M, Amin SA, Angell CA, Yarger JL (2016) NMR characterization of ionicity and transport properties for a series of diethylmethylamine based protic ionic liquids. J Phys Chem B 120:4279–4285. https://doi.org/10.1021/acs.jpcb.6b01203
Shmukler LE, Fedorova IV, Fadeeva YA, Gruzdev MS, Safonova LP (2022) Alkylimidazolium protic ionic liquids: structural features and physicochemical properties. ChemPhysChem 16:e202100772. https://doi.org/10.1002/cphc.202100772
Fedorova IV, Yablokov ME, Safonova LP (2022) Quantum-chemical study of acid-base interaction between alkylamines and different brønsted acids. Russ J Phys Chem A 96:2704–2711. https://doi.org/10.1134/S003602442212010X
Shmukler LE, Fedorova IV, Fadeeva YA, Safonova LP (2021) The physicochemical properties and structure of alkylammonium protic ionic liquids of RnH4-nNX (n=1–3) family. a mini-review. J Mol Liq 321:114350. https://doi.org/10.1016/j.molliq.2020.114350
Sun X, Cao B, Zhou X, Liu S, Zhu X, Fu H (2016) Theoretical and experimental studies on proton transfer in acetate-based protic ionic liquids. J Mol Liq 221:254–261. https://doi.org/10.1016/j.molliq.2016.05.080
Bodo E, Bonomo M, Mariani A (2021) Assessing the structure of protic ionic liquids based on triethylammonium and organic acid anions. J Phys Chem B 125:2781–2792. https://doi.org/10.1021/acs.jpcb.1c00249
Fedorova IV, Krestyaninov MA, Safonova LP (2017) Ab initio study of structural features and H-bonding in alkylammonium-based protic ionic liquids. J Phys Chem A 121:7675–7683. https://doi.org/10.1021/acs.jpca.7b05393
Palumbo O, Cimini A, Trequattrini F, Brubach J-B, Roy P, Paolone A (2020) The infrared spectra of protic ionic liquids: performances of different computational models to predict hydrogen bonds and conformer evolution. Phys Chem Chem Phys 22:7497–7506. https://doi.org/10.1039/D0CP00907E
Han J, Wang L, Zhang H, Su Q, Zhou X, Liu S (2020) Determinant factor for thermodynamic stability of sulfuric acid-amine complexes. J Phys Chem A 124:10246–10257. https://doi.org/10.1021/acs.jpca.0c07908
Chipanina NN, Aksamentova TN, Adamovich SN, Alabanov AI, Mirskova AN, Mirskov RG, Voronkov MG (2012) The proton transfer and hydrogen bonding complexes of (2-hydroxyethyl)amines with acids: a theoretical study. Comp Theor Chem 985:36–45. https://doi.org/10.1016/j.comptc.2012.01.033
Verma PL, Gejji SP (2018) Modeling protic dicationic ionic liquids based on quaternary ammonium, imidazolium or pyrrolidinium cations and bis(trifluoromethanesulfonyl)imide anion: structure and spectral characteristics. J Mol Graph Model 85:304–315. https://doi.org/10.1016/j.jmgm.2018.09.010
Fedorova IV, Shmukler LE, Fadeeva YA, Gruzdev MS, Safonova LP (2023) On structure and properties of tripropylammonium-based protic ionic liquids with bis(trifluoromethylsulfonyl)imide and hydrogen sulfate anions. Ionics 29:661–674. https://doi.org/10.1007/s11581-022-04844-5
Wang C, Guo L, Li H, Wang Y, Weng J, Wu L (2006) Preparation of simple ammonium ionic liquids and their application in the cracking of dialkoxypropanes. Green Chem 8:603–607. https://doi.org/10.1039/B600041J
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M et al (2009) Gaussian 09, revision A.01. Gaussian, Inc., Wallingford
Boys S, Bernardi F (2002) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Keith TA (2010) AIMAll, version 10.05.04 (aim.tkgristmill.com)
Glendening ED, Reed AE, Carpenter JE, Weinhold F (2013) NBO, version 3.1.
Dean JA (1998) Lange’s Handbook of Chemistry15th edn. McGraw-Hill, London
Covington AK, Thompson R (1974) Ionization of moderately strong acids in aqueous solution. Part III. Methane-, ethane-, and propanesulfonic acids at 25°C. J Solution Chem 3:603–617. https://doi.org/10.1007/BF00650404
Guthrie JP (1978) Hydrolysis of esters of oxy acids: pKa values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20 pK units. Can J Chem 56:2342–2354. https://doi.org/10.1139/v78-385
Low K, Tan SYS, Izgorodina EI (2019) An ab initio study of the structure and energetics of hydrogen bonding in ionic liquids. Front Chem 7:1–16. https://doi.org/10.3389/fchem.2019.00208
Bondi A (1964) Van der Waals volumes and radii. J Phys Chem 68:441–451. https://doi.org/10.1021/j100785a001
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P, Kjaergaard HG, Legon AC, Mennucci B, Nesbitt DJ (2011) Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl Chem 83:1637–1641. https://doi.org/10.1351/PAC-REC-10-01-02
Grabowski SJ (2006) Theoretical studies of strong hydrogen bonds. Annu Rep Prog Chem Sect C: Phys Chem 102:131–165. https://doi.org/10.1039/b417200k
Fedorova IV, Safonova LP (2020) Ion pair structures and hydrogen bonding in RnNH4-n alkylammonium ionic liquids with hydrogen sulfate and mesylate anions by DFT computations. J Phys Chem A 124:3170–3179. https://doi.org/10.1021/acs.jpca.0c01282
Bader RFW (1985) Atoms in molecules. Acc Chem Res 18:9–15. https://doi.org/10.1021/ar00109a003
Bader RFW (1990) Atoms in Molecules: A Quantum Theory. Oxford University Press, Oxford
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928. https://doi.org/10.1021/cr00005a013
Verma PL, Gejji SP (2019) Electronic structure and spectral characteristics of alkyl substituted imidazolium based dication-X2 (X=Br, BF4, PF6 and CF3SO3) complexes from theory. J Mol Liq 293:111548. https://doi.org/10.1016/j.molliq.2019.111548
Shakourian-Fard M, Fattahi A, Bayat A (2012) Ionic liquid based on α-amino acid anion and N7, N9- dimethylguaninium cation ([dMG][AA]): theoretical study on the structure and electronic properties. J Phys Chem A 116:5436–5444. https://doi.org/10.1021/jp211774y
Bader RFW, Essen HJ (1984) The characterization of atomic interactions. J Chem Phys 80:1943–1960. https://doi.org/10.1063/1.446956
Cremer D, Kraka E (1984) Chemical bonds without bonding electron density - does the difference electron-density analysis suffice for a description of the chemical bond? Angew Chem Int Ed Engl 23:627–628. https://doi.org/10.1002/anie.198406271
Weinhold F, Landis C (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. Cambridge University Press, New York
Weinhold F (1997) Nature of H-bonding in clusters, liquids, and enzymes: an ab initio, natural bond orbital perspective. J Mol Struct THEOCHEM 398:181–197. https://doi.org/10.1016/S0166-1280(96)04936-6
Fedorova IV, Krestyaninov MA, Safonova LP (2021) Structure and ion-ion interactions in trifluoroacetate-based ionic liquids: quantum chemical and molecular dynamics simulation studies. J Mol Liq 328:115449. https://doi.org/10.1016/j.molliq.2021.115449
Tsuzuki S, Shinoda W, Miran MS, Kinoshita H, Yasuda T, Watanabe M (2013) Interaction in ion pairs of protic ionic liquids: comparision with aprotic ionic liquids. J Chem Phys 139:174504. https://doi.org/10.1063/1.4827519
Grabowski SJ, Sokalski WA (2005) Different types of hydrogen bonds: correlation analysis of interaction energy components. J Phys Org Chem 18:779–784. https://doi.org/10.1002/poc.937
Keutsch FN, Cruzan JD, Saykally RJ (2003) The water trimer. Chem Rev 103:2533–2578. https://doi.org/10.1021/cr980125a
Munshi P, Row TNG (2005) Exploring the lower limit in hydrogen bonds: analysis of weak C− H...O and C− H... π interactions in substituted coumarins from charge density analysis. J Phys Chem A 109:659–672. https://doi.org/10.1021/jp046388s
Deshmukh MM, Gadre SR (2021) Molecular tailoring approach for the estimation of intramolecular hydrogen bond energy. Molecules 26:2928. https://doi.org/10.3390/molecules26102928
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. J Chem Phys Lett 285:170–173. https://doi.org/10.1016/S0009-2614(98)00036-0
Bartashevich EV, Nikulov DK, Vener MV, Tsirelson VG (2011) QTAIMC study of the X-H/H…O bond order indices (X = O, N, C) in molecular systems. Comput Theor Chem 973:33–39. https://doi.org/10.1016/j.comptc.2011.06.025
Bankiewicz B, Matczak P, Palusiak M (2012) Electron density characteristics in bond critical point (QTAIM) versus interaction energy components (SAPT): the case of charge-assisted hydrogen bonding. J Phys Chem A 116:452–459. https://doi.org/10.1021/jp210940b
Nikolaienko TY, Bulavin LA, Hovorun DM (2012) Bridging QTAIM with vibrational spectroscopy: the energy of intramolecular hydrogen bonds in DNA-related biomolecules. Phys Chem Chem Phys 14:7441–7447. https://doi.org/10.1039/C2CP40176B
Jabłonski M, Monaco G (2013) Different zeroes of interaction energies as the cause of opposite results on the stabilizing nature of C-H…O intramolecular interactions. J Chem Inf Model 53:1661–1675. https://doi.org/10.1021/ci400085t
Emamian S, Lu T, Kruse H, Emamian H (2019) Exploring nature and predicting strentgh of hydrogen bonds: a correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J Comput Chem 40:2868–2881. https://doi.org/10.1002/jcc.26068
Sanchora P, Pandey DK, Kagdada HL, Materny A, Singh DK (2020) Impact of alkyl chain length and water on the structure and properties of 1-alkyl-3-methylimidazolium chloride ionic liquids. Phys Chem Chem Phys 22:17687–17704. https://doi.org/10.1039/d0cp01686a
Agwupuye JA, Louis H, Unimuke TO, David P, Ubana EI, Moshood YL (2021) Electronic structure investigation of the stability, reactivity, NBO analysis, thermodynamics, and the nature of the interactions in methylsubstituted imidazolium-based ionic liquids. J Mol Liquids 337:116458. https://doi.org/10.1016/j.molliq.2021.116458
Jiang Y, Wanga Z, Lei Z, Yu G (2021) Structural effects on thermodynamic behavior and hydrogen bond interactions of water–ionic liquid systems. Chem Eng Sci 230:116186. https://doi.org/10.1016/j.ces.2020.116186
Li C, He H, Hou C, He M, Jiao C, Pan Q, Zhang M (2022) A quantum-chemistry and molecular-dynamics study o f non-covalent interactions between tri -n-butyl phosphate and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J Mol Liquids 360:119430. https://doi.org/10.1016/j.molliq.2022.119430
Guttmann R, Sax AF (2017) Dispersion interactions and the stability of amine dimers. ChemistryOpen 6:571–584. https://doi.org/10.1002/open.201700052
Izgorodina EI, MacFarlane DR (2011) Nature of hydrogen bonding in charged hydrogen-bonded complexes and imidazolium-based ionic liquids. J Phys Chem B 115:14659–14667. https://doi.org/10.1021/jp208150b
Ludwig R (2015) The effect of dispersion forces on the interaction energies and far infrared spectra of protic ionic liquids. Phys Chem Chem Phys 17:13790–13793. https://doi.org/10.1039/C5CP00885A
Fumino K, Fossog V, Stange P, Paschek D, Hempelmann R, Ludwig R (2015) Controlling the subtle energy balance in protic ionic liquids: dispersion forces complete with hydrogen bonds. Angew Chem Int Ed 54:2792–2795. https://doi.org/10.1002/anie.201411509
Wei Y, Xu T, Zhang X, Di Y, Zhang Q (2018) Thermodynamic properties and intermolecular interactions of a series of n-butylammonium carboxylate ionic liquids. J Chem Eng Data 63:4475–4483. https://doi.org/10.1021/acs.jced.8b00583
Markusson H, Belieres JP, Johansson P, Angell CA, Jacobsson P (2007) Prediction of macroscopic properties of protic ionic liquids by ab initio calculations. J Phys Chem A 111:8717–8723. https://doi.org/10.1021/jp072036k
Reid JESJ, Agapito F, Bernardes CES, Martins F, Walker AJ, Shimizu S, Minas da Piedade ME (2017) Structure–property relationships in protic ionic liquids: a thermochemical study. Phys Chem Chem Phys 19:19928–19936. https://doi.org/10.1039/c7cp02230a
Hayes R, Imberti S, Warr GG, Atkin R (2013) The nature of hydrogen bonding in protic ionic liquids. Angew Chem Int Ed 52:4623–4627. https://doi.org/10.1002/anie.201209273
Funding
This work was supported by the Russian Science Foundation (grant no. 22-23-01155).
Author information
Authors and Affiliations
Contributions
IF: all quantum chemical calculations, writing—original draft, validation, visualization, conceptualization, project administration, and funding acquisition. LS: writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
The submission of this work is according to the ethics followed by the journal.
Consent to participate
Participation was consensual.
Consent for publication
All authors consent to publish.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fedorova, I.V., Safonova, L.P. Proton transfer between sulfonic acids and various propylamines by density functional theory calculations. J Mol Model 29, 230 (2023). https://doi.org/10.1007/s00894-023-05624-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05624-2