Abstract
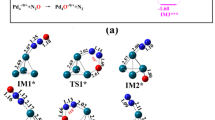
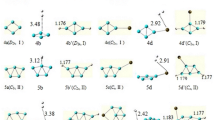
The adsorption and decomposition of N2O molecule over the surface of the MC23 clusters (M = Ru, Mn, V, Rh, and Pd) clusters were investigated using the dispersion-corrected PBE calculations (PBE-D3). The mechanism of the N2O dissociation over the above clusters has also been proposed, and the results show that the activation energies in PdC23, MnC23, VC23, RuC23, and RhC23 clusters are respectively 33.5, 7.9, 9.3, 24.4, and 42.4 kcal mol−1 in process 1 (TS1), and 68.5, 55.0, 55.3, 23.8, and 52.4 kcal mol−1 in process 2 (TS2). It was also demonstrated that the O2 desorption step in VC23 cluster was considered as the rate-limiting step, while the N2O decomposition step was found to be the rate-determining step for the other clusters. Moreover, the small desorption energies of O2 were obtained in RhC23, MnC23, RuC23, and PdC23 clusters, suggesting that the O2 desorption from the surface of the MC23 catalysts is easy; thereby, the regeneration of their surface metal sites is possible. This characteristic makes the MC23 (M = Mn, Rh, Pd, and Ru) clusters behave as highly active catalysts and therefore they could be potentially used as effective nanocatalysts for the N2O decomposition reaction.







Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within its supplementary material and from corresponding author on reasonable request.
References
Perez-Ramirez J, Kapteijin F, Schoffel K, Moulijn JA (2003) Formation and control of N2O in nitric acid production: where do we stand today? Appl Catal B 44:117–151
Kramlich JC, Linak WP (1994) Nitrous oxide behavior in the atmosphere, and in combustion and industrial systems. Prog Energy Combust Sci 20:149–202
Takoudis CG, Schmidt LD (1983) Kinetics of N2O decomposition on polycristalline Platinium. J Catal 80:274–279
Ramnani SP, Sabharwal S, Kumar JV, Reddy KHP, Rama Rao KS, Sai Prasad PS (2008) Advantage of radiolysis over impregnation method for the synthesis of SiO2 supported nano-Ag catalyst for direct decomposition of N2O. Catal Comm 9:756–761
Wei X, Yang X-F, Wang A-Q, Li L, Liu X-Y, Zhang T, Mou C-Y, Li J (2012) Bimetallic Au-Pd alloy catalysts for N2O decomposition : effects of surface structures on catalytic activity. J Phys Chem C 116:6222–6232
Pachatouridou E, Papista E, Delimitis A, Vasiliades MA, Efstathiou AM, Amiridis MD, Alexeev OS, Bloom D, Marnellos GE, Konsolakis M, Iliopoulou E (2016) N2O decomposition over ceria-promoted Ir/Al2O3 catalysts: The role of ceria. Appl Catal B 187:259–268
Carabineiro SA, Fernandes FB, Ramos AM, Vital JS, Silva IF (2000) Vanadium as a catalyst for NO and CO2 reactionwithactivatedcarbon. Catal Today 57:305–312
Zabilskiy M, Djinovic P, Erjavec B, Drazic G, Pintar A (2015) Small CuO clusters on CeO2 nanospheres as active species for catalytic N2O decomposition. Appl Catal B 163:113–122
Li HJ, Zheng L, Zhao ZT, Xu XF (2018) Effect of preparation parameters on the catalytic performance of hydrothermally synthesized Co3O4 in the decomposition of N2O. J Fuel Chem Technol 46:717–724
Sadovska G, Tabor E, Sazam P, Lhotka M, Bernauer M, Sobalik Z (2017) High temperature performenance and stability of Fe-FER catalyst for N2O decomposition. Catal Commun 89:133–137
Dou Z, Feng M, Xu XF (2013) Catalytic decomposition of N2O over Au/Co3O4 and Au/ZnCo2O4catalysts. J Fuel Chem Technol 41:1234–1240
Abu-Zied BM, Bawaked SM, Kosa SA, Ali TT, Schwieger W, Aqlan FM (2017) Effects of Nd-, Pr-, Tb- and Y-doping on the structural, textural, electrical and N2O decomposition activity of mesoporous NiO nanoparticles. Appl Surf Sci 419:399–408
Komvokis VG, Marnellos GE, Vasalos IA, Triantafyllidis KS (2009) Effect of pretreatment and regeneration conditions of Ru/γ-Al2O3 catalysts for N2O decomposition and/or reduction in O2-rich atmospheres and in the presence of NOX, SO2 and H2O. Appl Catal B 89:627–634
Zhu H, Li Y, Zheng X (2019) In-situ DRIFTS study of CeO2 supported Rh catalysts for N2O decomposition. Appl Catal A 571:89–95
Santiago M, Kondratenko VA, Kondratenko EV, López N, Ramírez JP (2011) Mechanistic analysis of direct N2O decomposition and reduction with H2 or NH3 over RuO2 Appl Catal B 110:33–39
Piumetti M, Hussain M, Fino D, Russo N (2015) Mesoporous silica supported Rh catalysts for high concentration N2O decomposition. Appl Catal B 165:158–168
Chromcakova Z, Obalova L, Kovanda F, Legut D, Titov A, Ritz M, Fridrichova D, Michalik S, Kustrowski P, Jiratova K (2015) Effect of precursor synthesis on catalyticactivity of Co3O4 in N2O decomposition. Catal Today 257:18–25
Xue L, Zhang C, He H, Teraoka Y (2007) Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl Catal B 75:167–174
da Cruz RS, Mascarenhas AJS, Andrade HMC (1998) Co-ZSM-5 catalysts for N2O decomposition. Appl Catal 18:223–231
Konkol M, Kondracka M, Kowalik P, Próchniak W, Michalska K, Schwedt A, Englert U (2016) Decomposition of the mixed-metal coordination polymer. A preparation route of the active Ag/Yb2O3catalyst for the de N2O process. Appl Catal B 190:85–92
Boissel V, Tahir S, Koh CA (2006) Catalytic decomposition of N2O over monolithic supported noble metal-transition metal oxides. Appl Catal B 64:234–242
Yuzaki K, Yarimizu T, Aoyagi K, Ito S-I, Kunimori K (1998) Catalytic decomposition of N2O over supported Rh catalysts: effects of supports and Rh dispersion. Catal Today 45:129–134
Kawi S, Liu SY, Shen S-C (2001) Catalytic decomposition and reduction of N2O on Ru/MCM-41 catalyst. Catal Today 68:237–244
Yamashita T, Vannice A (1996) N2O Decomposition over Manganese Oxides. J Catal 161:254–262
Komvokis VG, Marti M, Delimitis A, Vasalos IA, Triantafyllidis KS (2011) Catalytic decomposition of N2O over highly active supported Ru nanoparticles (≤ 3 nm) prepared by chemical reduction with ethylene glycol. Appl Catal B 103:62–71
Rodríguez-Kessler PL, Rodríguez-Domínguez AR (2015) N2O dissociation on small Rh clusters : A density functional study. Comput Mater Sci 97:32–35
Sun B-Z, Chen W-K, Wang X, Lu C-H (2007) A density functional theory study on the adsorption and dissociation of N2O on Cu2O (1 1 1) surface. Appl Surf Sci 253:7501–7505
Baei MT (2013) Si-doped B12N12 Nanocage as an Adsorbent for Dissociation of N2O to N2 Molecule. Hetero Chem 24:476–481
Lv Z, Mo H, Chen C, Ji X, Xu K, Miao L, Jiang J (2015) The effective adsorption and decomposition of N2O on Al-decorated graphene oxide under electric field. RSC Adv 24:1–25
Gholizadeh R, Yu Y-X, Wang Y (2017) N2O adsorption and decomposition over ZnO (0001) doped graphene: Density functional theory calculations. Appl Surf Sci 420:944–953
Zhang B, He G, Shan Y, He H (2019) Experimental and DFT study of the adsorption of N2O on transition ion-exchanged ZSM-5. Catal Today 327:177–181
Modak B, Srinivasu K, Ghosh SK (2017) Exploring metal decorated porphyrin-like porous fullerene as catalyst for oxygen reduction reaction: A DFT study. Int J Hydrogen Energy 42:2278–2287
Panahi Y, Sadeghi MM (2019) Application of metallofullerene towards adsorption of mustard gas: a detailed DFT study. J Inorg Organom Polym Mater 29:1383–1389
Zhang Y, Cheng X (2018) Hydrogen storage property of alkali and alkaline-earth metal atoms decorated C24 fullerene : A DFT study. Chem Phys 505:26–33
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J.A. Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision D.01. Gaussian, Inc., Wallingfor
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310
Krishnan RBJS, Binkley JS, Seeger R. Pople JA (1980) Self‐consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
Soltani A, Java MB, Baei MT, Azmoodeh Z (2018) Adsorption of chemical warfare agents over C24 fullerene: Effects of decoration of cobalt. J Alloys Compound 735:2148–2161
Tazikeh-Lemeski E, Soltani A, Baei MT, Bezi Javan M, Moazen Rad S (2018) Theoretical study on pure doped B12N12 fullerenes as thiophene sensor. Adsorption 24:585–593
Koopmans T (1934) Uber die zuordnung von wellenfunktionen und eigenwerten zu den einzelnen elektronen eines atoms. Physica 1:104–113
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764
Baei MT (2017) Benzene adsorption on C24, Si@C24, Si-doped C24 and C20 fullerenes. Russian J Phys Chem A 91:2530–2538
Derdare M, Boudjahem A, Boulbazine M (2021) Adsorption of the NO2, N2O and NH3 molecules over the C20 and MC19 (M = Ru, Ir and Au) clusters: A DFT approach. Surf Interfaces 24
Boulbazine M, Boudjahem A, Chaguetmi S, Karaman A (2020) Stability and electronic properties of Rh-doped ruthenium clusters and their interaction with NH3 molecule. Mol Phys 118
Gao X, Li Y, Chen J, Yang X, Zhang Z, Chang Z, Li Y (2021) First-principles study of N2O decomposition on (001) facet of perovskite LaBO3 (B = Mn, Co and Ni). Mol Catal 510
Wang AY, Wang YL, Walter ED, Kukkadapu RK, Gao YL, Lu GZ, Weber RS, Wang Y, Peden CHF, Gao F (2018) Catalytic N2O decomposition and reduction by NH3 over Fe/Beta and Fe/SSZ-13 catalysts. J Catal 358:199–210
Nobukawa T, Tanaka S, Itor S, Tomishige K, Kameoka S, Kunimori K (2002) Istopic study of N2O decomposition on an iron-exchange Fe-Zeolite catalyst: Mechanism of O2 formation. Catal Lett 83:5–8
Piskorz W, Zasada Z, Stelmachowski P, Diwald O, Kotarba A, Sojka Z (2011) Computational and experimental investigations into N2O decomposition over MgO nanocrystals from thorough molecular mechanism to ab initio micro-kinetics. J Phys Chem C 115:22451–22460
Song EH, Yan JM, Lian JS, Jiang Q (2012) External electric field catalyzed N2O decomposition on Mn-embedded graphene. J Phys Chem C 116:20342–20348
Gholizadeh R, Yu YX (2015) N2O + CO reaction over Si- and Se-doped graphenes: an ab initio DFT study. Appl Surf Sci 357:1187–1195
Wannakao S, Nongnual T, Khongpracha P, Maihom T, Limtrakul J (2012) Reaction mechanisms for CO catalytic oxidation by N2O on Fe-embedded graphene. J Phys Chem C 116:16992–16998
Vakili M, Gholizadeh R, Ghadi A, Salmasi E, Sinnokrot M (2020) Computational investigation of N2O adsorption and dissociation on the silicon-embedded graphene catalyst: A density functional theory. J Mol Graph Model 101
Liu X, Yang Z, LI Y, Zhang F (2015) Theoretical study of N2O decomposition mechanism over binuclear Cu-ZSM-5 zeolites. J Mol Catal A 396:81–187
Author information
Authors and Affiliations
Contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated competently in the work to take overall responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Abdel-Ghani Boudjahem: supervision, project administration, writing-original draft, writing- review and editing. Meryem Derdare: software, methodology. Mouhssin Boulbazine: conceptualization, investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Derdare, M., Boudjahem, AG. & Boulbazine, M. Adsorption and decomposition mechanism of N2O molecule over MC23 (M = Ru, Mn, V, Pd, and Rh) nanoclusters: A comparative DFT investigation. Struct Chem 33, 2043–2062 (2022). https://doi.org/10.1007/s11224-022-01984-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-01984-2