Abstract
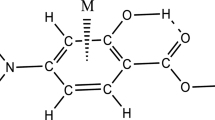
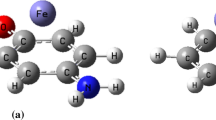
The interplay among two important noncovalent interactions involving aromatic ring is studied by means of density functional theory (DFT) calculations on complexes of methyl salicylate with Mn+, Fe2+, Co+, Ni2+, Cu+, and Zn2+ cations. The energetic, geometrical, spectroscopic, topological, and molecular orbital descriptors are applied to evaluate the strength of the cation-π and intramolecular hydrogen bond (IMHB) interactions. These outcomes are compared with the parent molecule of methyl salicylate and the corresponding results of benzene (BEN) complexes with the cited cations as a set of reference points. Based on the energetic conclusions, for the double-charge cations, the simultaneous presence of these interactions enhances the strength of the cation-π, while for the mono-charge cations, the reverse process is observed. On the other hand, for both type of the cations (mono- and double-charge), the coupling of noncovalent interactions reduces the strength of the IMHB in the studied systems. The computations in this study are discussed with the Bader theory of atoms in molecules (AIM), the natural bond orbital (NBO) analysis, and the frontier molecular orbital (FMO) theory.





Similar content being viewed by others
Data availability
From corresponding authors upon request.
References
Gerhartz W (1985) Ullmann’s encyclopedia of industrial chemistry. VCH, Hoboken
Carson JL, Willett LR (1993) Toxicity of nonsteroidal anti-inflammatory drugs. An overview of the epidemiological evidence. Drugs 46:243–248. https://doi.org/10.2165/00003495-199300461-00063
Mason L, Moore RA, Edwards JE, McQuay HJ, Derry S, Wiffen PJ (2004) Systematic review of efficacy of topical rubefacients containing salicylates for the treatment of acute and chronic pain. BMJ 328:995. https://doi.org/10.1136/bmj.38040.607141.EE
Vaile JH, Davis P (1998) Topical NSAIDs for musculoskeletal conditions. A review of the literature. Drugs 56:783–799. https://doi.org/10.2165/00003495-199856050-00004
Meyer EA, Castellano RK, Diederich F (2003) Interactions with aromatic rings in chemical and biological recognition. Angew Chem Int Ed 42:1210–1250. https://doi.org/10.1002/anie.200390319
Dougherty DA (1996) Cation-π interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Science 271:163–168. https://doi.org/10.1126/science.271.5246.163
Kim KS, Tarakeshwar P, Lee JY (2000) Molecular clusters of π-systems: theoretical studies of structures, spectra, and origin of interaction energies. Chem Rev 100:4145–4186. https://doi.org/10.1021/cr990051i
Lee EC, Kim D, Juree’ka P, Tarakeshwar P, Hobza P, Kim KS (2007) Understanding of assembly phenomena by aromatic−aromatic interactions: benzene dimer and the substituted systems. J Phys Chem A 111:3446–3457. https://doi.org/10.1021/jp068635t
Reddy AS, Sastry GN (2005) Cation [M = H+, Li+, Na+, K+, Ca2+, Mg2+, NH4+, and NMe4+] interactions with the aromatic motifs of naturally occurring amino acids: a theoretical study. J Phys Chem A 109:8893–8903. https://doi.org/10.1021/jp0525179
E’erný J, Hobza P (2007) Non-covalent interactions in biomacromolecules. Phys Chem Chem Phys 9:5291–5303. https://doi.org/10.1039/B704781A
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biology and chemistry. Springer-Verlag, Berlin
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, Oxford
Scheiner S (1997) Hydrogen bonding. A Theoretical Perspective. Oxford University Press, Oxford
Pauling L (1960) The nature of the chemical bond. Cornell University Press, Ithaca, New York
Buckingham AD, Legon AC, Roberts SM (1993) Principles of molecular recognition. Blackie Academic & Professional, London
Gilli G, Belluci F, Ferretti V, Bertolasi V (1989) Evidence for resonance-assisted hydrogen bonding from crystal-structure correlations on the enol form of the .beta.-diketone fragment. J Am Chem Soc 111:1023–1028. https://doi.org/10.1021/ja00185a035
Cubero E, Luque FJ, Orozco M (1998) Is polarization important in cation–π interactions? Proc Natl Acad Sci 95:5976–5980. https://doi.org/10.1073/pnas.95.11.5976
Ma JC, Dougherty DA (1997) The Cation−π interaction. Chem Rev 97:1303–1324. https://doi.org/10.1021/cr9603744
Schneider HJ (1991) Mechanisms of molecular recognition: investigations of organic host–guest complexes. Angew Chem Int Ed Eng 30:1417–1436. https://doi.org/10.1002/anie.199114171
Hong BH, Bae SC, Lee CW, Jeong S, Kim KS (2001) Ultrathin single-crystalline silver nanowire arrays formed in an ambient solution phase. Science 294:348–351. https://doi.org/10.1126/science.1062126
Choi HS, Suh SB, Cho SJ, Kim KS (1998) Ionophores and receptors using cation-π interactions: collarenes. Proc Natl Acad Sci U S A 95:12094–12099. https://doi.org/10.1073/pnas.95.21.12094
Kim D, Tarakeshwar P, Kim KS (2004) Theoretical investigations of anion−π interactions: the role of anions and the nature of π systems. J Phys Chem A 108:1250–1258. https://doi.org/10.1021/jp037631a
Kim D, Hu S, Tarakeshwar P, Kim KS (2003) Cation−π interactions: a theoretical investigation of the interaction of metallic and organic cations with alkenes, arenes, and heteroarenes. J Phys Chem A 107:1228–1238. https://doi.org/10.1021/jp0224214
Hong BH, Lee JY, Lee CW, Kim JC, Bae SC, Kim KS (2001) Self-assembled arrays of organic nanotubes with infinitely long one-dimensional H-bond chains. J Am Chem Soc 123:10748–10749. https://doi.org/10.1021/ja016526g
Kim KS, Lee JY, Ha TK, Kim DH (1994) On binding forces between aromatic ring and quaternary ammonium compound. J Am Chem Soc 116:7399–7400. https://doi.org/10.1021/ja00095a050
Hunter CA, Sanders JKM (1990) The nature of π-π interactions. J Am Chem Soc 112:5525–5534. https://doi.org/10.1021/ja00170a016
Guo H, Salahub DR (1998) Cooperative hydrogen bonding and enzyme catalysis. Angew Chem Int Ed 37:2985–2990. https://doi.org/10.1002/(SICI)1521-3773(19981116)37:21<2985::AID-ANIE2985>3.0.CO;2-8
Estarellas C, Escudero D, Frontera A, Quiñonero D, Deyá PM (2009) Theoretical ab initio study of the interplay between hydrogen bonding, cation–π and π–π interactions. Theor Chem Accounts 122:325–332. https://doi.org/10.1007/s00214-009-0517-0
Estarellas C, Frontera F, Quiñonero D, Deyá PM (2009) Interplay between cation–π and hydrogen bonding interactions: are non-additivity effects additive? Chem Phys Lett 479:316–320. https://doi.org/10.1016/j.cplett.2009.08.035
Escudero D, Frontera A, Quiñonero D, Deyá PM (2008) Interplay between cation-π and hydrogen bonding interactions. Chem Phys Lett 456:257–261. https://doi.org/10.1016/j.cplett.2008.03.028
Vijay D, Zipse H, Narahari Sastry G (2008) On the cooperativity of cation-π and hydrogen bonding interactions. J Phys Chem B 112:8863–8867. https://doi.org/10.1021/jp804219e
Li Q, Li W, Cheng J, Gong B, Sun J (2008) Effect of methyl group on the cooperativity between cation–π interaction and NH···O hydrogen bonding. J Mol Struct 867:107–110. https://doi.org/10.1016/j.theochem.2008.07.031
Zakian VA (1995) Telomeres: beginning to understand the end. Science 270:1601–1607. https://doi.org/10.1126/science.270.5242.1601
Rooman M, Lievin J, Bulsine E, Wintjens R (2002) Cation–π/H-bond stair motifs at protein–DNA interfaces. J Mol Biol 319:67–76. https://doi.org/10.1016/s0022-2836(02)00263-2
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann JRE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, HeadGordon M, Replogle ES, Pople JA (2003) Gaussian 03, revision B.01. Gaussian, Inc, Pittsburgh
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements. Theor Chem Accounts 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269. https://doi.org/10.1063/1.447079
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, New York
Biegler-König FW, Bader RFW, Tang TH (1982) Calculation of the average properties of atoms in molecules. II. J Comput Chem 3:317–328. https://doi.org/10.1002/jcc.540030306
Biegler-König FW, Schonbohm J, Derdan R, Bayles D, Bader R (2000) AIM2000, Version 2.000
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/cr00088a005
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1992) NBO, version 3.1. Gaussian Inc., Pittsburgh
Pearson RG (1997) Chemical hardness – applications from molecules to solids. Weinheim, VCH-Wiley
Chattaraj PK, Poddar A (1999) Molecular reactivity in the ground and excited electronic states through density-dependent local and global reactivity parameters. J Phys Chem A 103:8691–8699. https://doi.org/10.1021/jp991214+
Parr RG, Lv S, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Sen KD, Jorgensen CK (1987) Electronegativity, structure and bonding. Springer Verlag, New York
Koopmans T (1934) Uber die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines atoms. Physica 1:104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Espinosa E, Molins E (2000) Retrieving interaction potentials from the topology of the electron density distribution: the case of hydrogen bonds. J Chem Phys 113:5686–5694. https://doi.org/10.1063/1.1290612
Espionsa E, Souhassou M, Lachekar H, Lecomte C (1999) Topological analysis of the electron density in hydrogen bonds. Acta Crystallogr B 55:563–572. https://doi.org/10.1107/s0108768199002128
Abramov YA (1997) On the possibility of kinetic energy density evaluation from the experimental electron-density distribution. Acta Crystallogr A 53:264–272. https://doi.org/10.1107/S010876739601495X
Palusiak M, Simon S, Sola M (2006) Interplay between intramolecular resonance-assisted hydrogen bonding and aromaticity in o-hydroxyaryl aldehydes. J Organomet Chem 71:5241–5248. https://doi.org/10.1021/jo060591x
Güell G, Poater J, Luis JM, Mó O, Yáñez M, Sola M (2005) Aromaticity analysis of lithium cation/π complexes of aromatic systems. Chem Phys Chem 6:2552–2561. https://doi.org/10.1002/cphc.200500216
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41:48–76. https://doi.org/10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U
Garau C, Frontera A, Quiñonero D, Ballester P, Costa A, Deyà PM (2004) Cation-π versus anion-π interactions: energetic, charge transfer, and aromatic aspects. J Phys Chem A 108:9423–9427. https://doi.org/10.1021/jp047534x
Parra RD, Ohlssen J (2008) Cooperativity in intramolecular bifurcated hydrogen bonds: an ab initio study. J Phys Chem A 112:3492–3498. https://doi.org/10.1021/jp711956u
Ziółkowski M, Grabowski SJ, Leszczynski J (2006) Cooperativity in hydrogen-bonded interactions: ab initio and “atoms in molecules” analyses. J Phys Chem A 110:6514–6521. https://doi.org/10.1021/jp060537k
Balachandran V, Nataraj A, Karthick T (2013) Molecular structure, spectroscopic (FT-IR, FT-Raman) studies and first-order molecular hyperpolarizabilities, HOMO–LUMO, NBO analysis of 2-hydroxy-p-toluic acid. Spectrochim Acta A Mol Biomol Spectrosc 104:114–129. https://doi.org/10.1016/j.saa.2012.11.052
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21:748(1–22). https://doi.org/10.3390/molecules21060748
Baeten A, Proft FD, Geerlings P (1995) Basicity of primary amines: a group properties based study of the importance of inductive (electronegativity and softness) and resonance effects. Chem Phys Lett 235:17–21. https://doi.org/10.1016/0009-2614(95)00084-H
Baeten A, Proft FD, Geerlings P (1996) Proton affinity of amino acids: their interpretation with density functional theory-based descriptors. Int J Quantum Chem 60:931–939. https://doi.org/10.1002/(SICI)1097-461X(1996)60:4<931::AID-QUA14>3.0.CO;2-7
Akher FB, Ebrahimi A (2015) π-Stacking effects on the hydrogen bonding capacity of methyl 2-naphthoate. J Mol Graph Model 61:115–122. https://doi.org/10.1016/j.jmgm.2015.06.013
Kushwaha PS, Mishra PC (2000) Relationship of hydrogen bonding energy with electrostatic and polarization energies and molecular electrostatic potentials for amino acids: an evaluation of the lock and key model. Int J Quantum Chem 76:700–713. https://doi.org/10.1002/(SICI)1097-461X(2000)76:6<700::AID-QUA3>3.0.CO;2-V
Mishra PC, Kumar A (1996) Molecular electrostatic potentials and fields: hydrogen bonding, recognition, reactivity and modeling. Theor Comput Chem 3:257–296. https://doi.org/10.1016/S1380-7323(96)80046-X
Acknowledgments
The support of this work by Vali-e-Asr University of Rafsanjan is acknowledged.
Author information
Authors and Affiliations
Contributions
M. P. is a graduate student who prepared the complexes and worked on the structures under direct supervision of M. M.; A. K. is an advisor, and M. M. wrote the manuscript.
Corresponding author
Ethics declarations
Not applicable. The ethical standards have been met.
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Gaussian 03 Revision-B.01-SMP.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pirgheibi, M., Mohammadi, M. & Khanmohammadi, A. A comparative study of interplay effects between the cation-π and intramolecular hydrogen bond interactions in the various complexes of methyl salicylate with Mn+, Fe2+, Co+, Ni2+, Cu+, and Zn2+ cations. Struct Chem 32, 1529–1539 (2021). https://doi.org/10.1007/s11224-021-01728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01728-8