Abstract
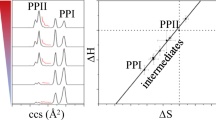
Two procyanidin B2 conformers were identified in a relative abundance ratio of approximately 3:1 at 298 K by 1H NMR experiments in acetonitrile. The conformational interchange reactions between these two conformers are 1st order in both reactions, with ∆G‡ for forward and reverse of 57.12 ± 5.62 and 54.56 ± 5.48 kJ mol−1, respectively. The experimentally obtained standard thermodynamic energies for this reaction are ΔH0rxn (3.67 ± 0.22 kJ mol−1), ΔS0rxn (4.05 ± 1.57 kJ mol−1 K−1), and ΔH0rxn (2.96 ± 0.33 kJ.mol−1). Conformational search results at the DFT (PBE, PBE-D2, and B3LYP with 6-311++g**) level of theory yielded four novel conformations, named fully compact (FC), partially compact (PC), partially extended (PE), and fully extended (FE). Although the FC conformer is electronically the most stable of the four as a result of extensive intramolecular non-covalent interactions, the PC and FE conformers are thermodynamically favored in a 5:1 ratio (B3LYP), with the FC and PE conformers present in negligible amounts at equilibrium. The DFT computed standard reaction energies using the B3LYP functional for the PCmajor to FEminor conformational interchange reaction compare exceptionally well with experimental data at 298 K: ∆G0rxn (3.86 kJ mol−1), ΔH0rxn (5.34 kJ mol−1), and ∆S0rxn (4.97 kJ mol−1 K−1). It was found that inclusion of solvation energies is crucial to obtain accurate thermodynamic energies. The secondary equilibria found in chromatographic separations are predicted to be highly dependent on solvent polarity and temperature. Similar conformational diversity and hierarchies should exist for all non-rigid procyanidin oligomers and the unique chromatographic behavior of these compounds may be a result of conformational interchange.











Similar content being viewed by others
References
Bader RFW, Tal Y, Anderson SG, Nguyen-Dang TT (1980) Quantum topology: theory of molecular structure and its change. Isr J Chem 19(1–4):8–29
Bader RFW, Nguyen-Dang TT, Tal Y (1981) A topological theory of molecular structure. Rep Prog Phys 44(8):893
Becke A (1993) B3LYP. J Chem Phys 98:5648–5652
Bloch F (1946) Nuclear induction. Phys Rev 70(7–8):460–474
Cala O, Pinaud N, Simon C, Fouquet E, Laguerre M, Dufourc EJ, Pianet I (2010) NMR and molecular modeling of wine tannins binding to saliva proteins: revisiting astringency from molecular and colloidal prospects. FASEB J 24(11):4281–4290
Cala O, Fabre S, Pinaud N, Dufourc EJ, Fouquet E, Laguerre M, Pianet I (2011) Towards a molecular interpretation of astringency: synthesis, 3D structure, colloidal state, and human saliva protein recognition of procyanidins. Planta Med 77:1116–1122
Cala O, Dufourc EJ, Fouquet E, Manigand C, Laguerre M, Pianet I (2012) The colloidal state of tannins impacts the nature of their interaction with proteins: the case of salivary proline-rich protein/procyanidins binding. Langmuir 28(50):17410–17418
De Gaulejac NSC, Provost C, Vivas N (1999) Comparative study of polyphenol scavenging activities assessed by different methods. J Agric Food Chem 47(2):425–431
de Villiers A, Cabooter D, Lynen F, Desmet G, Sandra P (2009) High performance liquid chromatography analysis of wine anthocyanins revisited: effect of particle size and temperature. J Chromatogr A 1216(35):3270–3279
Esatbeyoglu T, Jaschok-Kentner B, Wray V, Winterhalter P (2011) Structure elucidation of procyanidin oligomers by low-temperature 1H NMR spectroscopy. J Agric Food Chem 59:62–69
Foo LY, Porter LJ (1983) Synthesis and conformation of procyanidin diastereoisomers. J Chem Soc Perkin Trans. https://doi.org/10.1039/p19830001535
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenber DJ (2009) Gaussian 09. Gaussian, Inc, Wallingford, pp 2–3
Grimme S (2011) Density functional theory with London dispersion corrections. Wiley Interdiscip Rev Comp Mol Sci 1(2):211–228
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys. https://doi.org/10.1063/1.3382344
Hammerstone JF, Lazarus SA, Mitchell AE, Rucker R, Schmitz HH (1999) Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography / mass spectrometry. J Agric Food Chem 47:490–496
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53(6):1841–1856
Kalili KM, de Villiers A (2013) Systematic optimisation and evaluation of on-line, off-line and stopflow comprehensive hydrophilic interaction chromatography x reversed phase liquid chromatographic analysis of procyanidins. Part II: application to cocoa procyanidins. J Chromatogr A 1289:69–79
Kalili KM, Cabooter D, Desmet G, de Villiers A (2012) Kinetic optimisation of the reversed phase liquid chromatographic separation of proanthocyanidins on sub-2 m and superficially porous phases. J Chromatogr A 1236(63):63–76
Khan M, Haslam E, Williamson MP (1997) Structure and conformation of the procyanidin B-2 dimer. Magn Reson Chem 35:854–858
Kothe L, Zimmermann BF, Galensa R (2013) Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem 141(4):3656–3663
Mathews JH, Fink KK (2004) Numerical methods using Matlab. Pearson, New Jearsey
McConnell HM (1958) Reaction rates by nuclear magnetic resonance. J Chem Phys. https://doi.org/10.1063/1.1744152
Mendoza-Wilson AM, Castro-arredondo SI, Balandran-quintana RR (2014) Computational study of the structure free radical scavenging relationship of procyanidins. Food Chem 161:155–161
Murphy KJ, Chronopoulos AK, Singh I, Francis MA, Moriarty H, Pike MJ, Turner AH, Mann NJ, Sinclair AJ (2003) Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr 77(6):1466–1473
Pianet I, Andre Y, Ducasse MA, Tarascou I, Lartigue JC, Pinaud N, Fouquet E, Dufourc EJ, Laguerre M (2008) Modeling procyanidin self-association processes and understanding their micellar organization: a study by diffusion NMR and molecular mechanics. Langmuir 24(19):11027–11035
Pirker KF, Oliveira J, de Freitas V, Goodman BA, Mateus N (2011) Antiradical properties of red wine portisins. J Agric Food Chem 59(21):11833–11837
Pople JA, Segal GA (1965) Approximate self-consistent molecular orbital theory. II. Calculations with complete neglect of differential overlap. J Chem Phys 43(10):S136–S151
Pople JA, Santry DP, Segal GA (1965) Approximate self-consistent molecular orbital theory. I. Invariant procedures. J Chem Phys 43(10):S129–S135
Schmidt CA, Murillo R, Heinzmann B, Laufer S, Wray V, Merfort I (2011) Structural and conformational analysis of proanthocyanidins from parapiptadenia rigida and their wound-healing properties. J Nat Prod 74:1427–1436
Shahat AA (2006) Procyanidins from Adansonia digitata. Pharm Biol 44(6):445–450
Tarascou I, Barathieu K, Simon C, Ducasse MA, Andre Y, Fouquet E, Dufourc EJ, de Freitas V, Laguerre M, Pianet I (2006) A 3D structural and conformational study of procyanidin dimers in water and hydro-alcoholic media as viewed by NMR and molecular modeling. Magn Reson Chem 44(9):868–880
Wanfei CAI, Shuang MAO, Shu Z, Fei CAO, Hong Z, Laicai LI, Anmin T (2011) Density functional theory study on the interaction of catechin and cytosine. Sci China B 54(7):1094–1100
Xu J, Rong S, Xie B, Sun Z, Zhang L, Wu H, Yao P, Hao L, Liu L (2010) Procyanidins extracted from the lotus seedpod ameliorate age-related antioxidant deficit in aged rats. J Gerontol A 65(3):236–241
Zhang Y, Kong L, Yin C, Jiang D, Jiang J, He J, Xiao W (2013) Extraction optimization by response surface methodology, purification and principal antioxidant metabolites of red pigments extracted from bayberry (Myrica rubra) pomace. J Food Sci Technol 51(1):343–347
Funding
The authors received financial support from SASOL (Collaborative grant to AdV), and the National Research Foundation (NRF, grant 98897 to AdV).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All ethical guidelines have been adhered to.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 1416 kb)
Rights and permissions
About this article
Cite this article
O’Kennedy, S.J., de Villiers, A., Brand, D.J. et al. A variable temperature 1H NMR and DFT study of procyanidin B2 conformational interchange. Struct Chem 29, 1551–1564 (2018). https://doi.org/10.1007/s11224-018-1153-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1153-x