Abstract
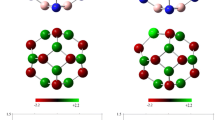
The adsorption properties of the phenol molecule (C6H5OH) upon the outer surfaces of C24, B12P12, B12N12, Al12N12, and Al12P12 were investigated using density functional theory calculations. Our calculations reveal that the phenol molecule can be chemisorbed on the sidewalls of Al12N12 and Al12P12 with adsorption energies of −1.03 and −0.76 eV, respectively. While the adsorption energy of C6H5OH on Al12N12 is typically more than that of Al12P12 cluster. We also considered the adsorption of the C6H5OH molecule under a strong electric field over Al12N12. The results indicate that Al12N12 has high sensitivity to the phenol molecule in the presence of an electric field.





Similar content being viewed by others
References
Wallace J (2005) Kirk-Othmer, encyclopedia of chemical technology, vol 18, 3rd edn. Wiley, New York, p 747
Budavari S (1996) The Merck Index: an encyclopedia of chemical, drugs, and biologicals. Merck, Whitehouse Station
Lin TM, Lee SS, Lai CS, Lin SD (2006) J Int Soc Burn Inj 32:517
Warner MA, Harper JV (1985) Anesthesiology 62:366
Hanscha C, McKarnsb SC, Smith CJ, Doolittle DJ (2000) Chem Biol Interact 127:61
Oku T, Hirano T, Kuno M, Kusunose T, Niihare K, Suganuma K (2000) Mater Sci Eng B 74:217
Oku T, Nishiwaki A, Narita I (2004) Sci Technol Adv Mater 5:635
Wang H (2010) Chin J Chem 28:1897
Sabirov DS, Bulgakov RG (2011) Comput Theor Chem 963:185
Zhao J-x, Gao B, Cai Q-h, Wang X-g, Wang X-z (2011) Theor Chem Acc 129:85
Chakarova-Käck SD, Øyvind B, Schröder E, Lundqvist BI (2006) Phys Rev B 74:155402
Delle Site L, Alavi A, Abrams CF (2003) Phys Rev B 67:193406
Myers AK, Benziger JB (1989) Langmuir 5:1270
Xu X, Friend CM (1989) J Phys Chem 93:8072
Ihm H, White JM (2000) J Phys Chem B 104:6202
Ghiringhelli LM, Caputo R, Delle Site L (2007) Phys Rev B 75:113403
Beheshtian J, Bagheri Z, Kamfiroozi M (2011) Microelectron J 42:1400
Ahmadi Phyghan A, Pashangpour M, Bagheri Z, Kamfiroozi M (2012) Physica E 44:1436
Beheshtian J, Kamfiroozi M, Bagheri Z, Ahmadi (2012) Comput Mater Sci 54:115
Beheshtian J, Peyghan AA, Bagheri Z (2012) Comput Mater Sci 62:71
Beheshtian J, Ahmadi Peyghan A, Bagheri Z, Kamfiroozi M (2012) Struct Chem 23:1567
Schmidt M, Baldridge K, Boatz J, Elbert S, Gordon M, Jensen J, Koseki S, Matsunaga N, Nguyen K, Su S, Windus T, Dupuis M (1993) J Comput Chem 14:1347
Beheshtian J, Baei MT, Ahmadi Peyghan A (2012) Surf Sci 606:981
Farmanzadeh D, Amirazami A (2009) Acta Phys Chim Sin 25:001
Farmanzadeh D, Ghazanfary S (2009) Struct Chem 20:709
Parr RG, Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512
Soltani A, Taghartapeh MR, Mighani H, Pahlevani AA, Mashkoor R (2012) Appl Surf Sci 259:637
Soltani A, Ahmadian N, Kanani Y, Dehno khalajid A, Mighani H (2012) Appl Surf Sci 258:9536
Baei MT, Ahmadi Peyghan A, Bagheri Z (2013) Solid State Commun 159:8
Acknowledgments
We would like to thank the Jaber Ebne Hayyan Unique Industry researchers Company. We should also thank the Nanotechnology Working Group of Young Researchers and Elite Club of Islamic Azad University, Gorgan Branch, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soltani, A., Baei, M.T., Taghartapeh, M.R. et al. Phenol interaction with different nano-cages with and without an electric field: a DFT study. Struct Chem 26, 685–693 (2015). https://doi.org/10.1007/s11224-014-0504-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0504-5