Abstract
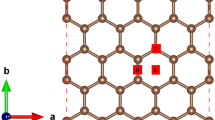
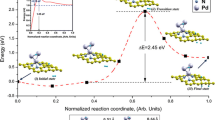
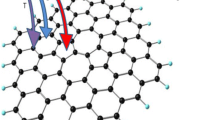
Employing density functional calculations including an empirical dispersion term, we investigated the hydrogenation of an aluminum nitride nanosheet (h-AlN) with atomic and molecular hydrogen. It was found that atomic H prefers to be adsorbed on an N atom rather than Al, releasing energy of 21.1 kcal/mol. The HOMO/LUMO energy gap of the sheet is dramatically reduced from 107.9 to 44.5 kcal/mol, upon the adsorption of one hydrogen atom. The adsorption of atomic H on the h-AlN presents properties which are promising for nanoelectronic applications. The molecular H2 was found to be adsorbed collinearly on an N atom and dissociated to two H atoms on Al–N bond. Calculated barrier and adsorption energies for this dissociation process are about +18.9 and −1.9 kcal/mol. We predict that each nitrogen atom in an AlN sheet can adsorb two hydrogen molecules on opposite sides of the sheet, and thus the gravimetric density for hydrogen storage on AlN sheet is evaluated to be about 8.9 wt%.







Similar content being viewed by others
References
Zabaniotou A, Ioannidou O, Antonakou E, Lappas A (2008) Int J Hydrogen Energy 33:2433
Kim BJ, Lee YS, Park SJ (2008) Int J Hydrogen Energy 33:2254
Makaka G, Meyer EL, McPherson M (2008) Renew Energy 33:1959
Banapurmath NR, Tewari PG, Hosmath RS (2008) Renew Energy 33:1982
Kanoglu M, Dincer I, Rosen MA (2007) Int J Hydrogen Energy 32:4250
Abbasi T, Abbasi SA (2011) Renew Sust Energ Rev 15:3034
Bao QX, Zhang H, Gao SW, Li XD, Cheng XL (2010) Struct Chem 21:1111
Dinadayalane TC, Leszczynski J (2010) Practical Aspects of Computational Chemistry. Springer, Netherlands, pp 297–313
Dinadayalane TC, Kaczmarek A, Lukaszewicz J, Leszczynski J (2007) J Phys Chem C 111:7376
Kaczmarek A, Dinadayalane TC, Lukaszewicz J, Leszczynski J (2007) Int J Quantum Chem 107:2211
Dinadayalane TC, Leszczynski J (2007) Chem Phys Lett 434:86
Dinadayalene TC, Murray JS, Concha MC, Politzer P, Leszczynski J (2010) J Chem Theory Comput 6:1351
Bagheri Z, Moradi M (2013) Struct Chem. doi:10.1007/s11224-013-0321-2
Beheshtian J, Bagheri Z, Kamifiroozi M, Ahmadi A (2012) Struct Chem 23:653
Wu XJ, Yang JL, Zeng XC (2006) J Chem Phys 125:044704
Gil A, Trujillano R, Vicente MA, Korili SA (2009) Int J Hydrogen Energy 34:8611
Chan SP, Chen G, Gong XG, Liu ZF (2001) Phys Rev Lett 87:205502
Fu Q, Negro E, Chen G, Law DC, Li CH, Hicks RF, Raghavachari K (2002) Phys Rev B 65:075318
Schailey R, Ray AK (2000) Comput Mater Sci 22:169
Mei Y, Thurmer DJ, Deneke C, Kiravittaya S, Chen YF, Dadgar A, Bertram F, Bastek B, Krost A, Christen J (2009) ACS Nano 3:1663
Wang Q, Sun Q, Jena P, Kawazoe Y (2009) ACS Nano 3:621
Liu C, Hu Z, Wu Q, Wang X, Chen Y, Sang H, Zhu J, Deng S, Xu N (2005) J Am Chem Soc 127:1318
Du A, Zhu Z, Chen Y, Lu G, Smith SC (2009) Chem Phys Lett 469:183
Ma Y, Huo K, Wu Q, Lu Y, Hu Y, Hu Z, Chen Y (2006) J Mater Chem 16:2834
Li Y, Shen P, Zhang SB, Chen Z (2009) Nanotechnology 20:215701
Zhang X, Liu Z, Hark S (2007) Solid State Commun 143:317
de Almeida E, de Brito Mota F, de Castilho CMC, Kakanakova-Georgieva A, Gueorguiev G (2012) Eur Phys J B 85:1
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
O’Boyle NM, Tenderholt AL, Langner KM (2008) J Comput Chem 29:839
Xu S, Wang C, Cui Y (2010) Struct Chem 21:519
Paukku Y, Michalkova A, Leszczynski J (2008) Struct Chem 19:307
Wang C, Xu S, Ye L, Lei W, Cui Y (2010) Struct Chem 21:1215
Moradi M, Peyghan AA, Bagheri Z, Kamifiroozi M (2012) J Mol Model 18:3535
Rastegar SF, Peyghan AA, Ghenaatian HR, Hadipour NL (2013) Appl Surf Sci 274:217
Yang RT (2000) Carbon 38:623
McAfee JL, Poirier B (2009) J Chem Phys 130:064701
Ruffieux P, Gröning O, Bielmann M, Mauron P, Schlapbach L, Gröning P (2002) Phys Rev B 66:245416
Lin F, Zhou G, Li Z, Li J, Wu J, Duan W (2009) Chem Phys Lett 475:82
Lim SH, Lin J (2008) Chem Phys Lett 466:197
Han SS, Kang JK, Lee HM, Van Duin AC, Goddard WA III (2005) J Chem Phys 123:114704
Graetz J, Chaudhuri S, Lee Y, Vogt T, Muckerman JT, Reilly JJ (2006) Phys Rev B 74:214114
Dresselhaus MS (2004) Basic Research Needs for the Hydrogen Economy, 2nd edn. Office of Basic Energy Sciences, U.S. Department of Energy, Washington, DC
Varin RA, Czujko T, Chiu C, Wronski Z (2006) J Alloys Compd 424:356
Bhatia SK, Myers AL (2006) Langmuir 22:1688
Liu C, Fan YY, Liu M, Cong HT, Cheng HM, Dresselhaus MS (1999) Science 286:1127
Chen JP, Yang RT (1989) Surf Sci 216:481
Shevlin SA, Guo ZX (2007) Phys Rev B 76:024104
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moradi, M., Naderi, N. First principle study of hydrogen storage on the graphene-like aluminum nitride nanosheet. Struct Chem 25, 1289–1296 (2014). https://doi.org/10.1007/s11224-014-0410-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0410-x