Abstract
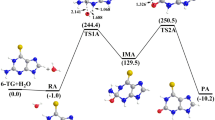
The mechanism of one-carbon unit transfer reaction between tetrahydrofolate coenzymes model compound (e.g., benzimidazolium) and Grignard reagent has been investigated employing the DFT and B3LYP/6-31G* levels of theory. Three consecutive reactions leading to major products N,N′-dimethyl-ophenylenediamine and acetone have been proposed and discussed. For these reactions, the structure parameters, vibrational frequencies, and energies for each stationary point have been calculated, and the corresponding reaction mechanism has been given by the potential energy surface, which is drawn according to the relative energies. The calculated results show that the corresponding major products N,N′-dimethyl-ophenylenediamine and acetone are in agreement with experimental findings, which provided a new illustration and guidance for these reactions.






Similar content being viewed by others
References
Kompis IM, Islam K, Then RL (2005) Chem Rev 105:593–620
Bieräugel H, Plemp R, Hiemstra HC, Pandit UK (1983) Tetrahedron 39:3971–3979
Huizenga RH, Vawiltenburg J, Bieräugel H, Pandit UK (1991) Tetrahedron 47:4165–4174
Bieräugel H, Brands KMJ, Pandit UK (1988) Heterocycles 27:1589–1593
Kulkowit S, McKervey MA (1978) J Chem Soc Chem Commun 23:1069–1070
Meyers AI, Collingt EW (1970) J Am Chem 92:6676–6678
Jiang JL, Shi Z (1998) Synth Commun 28:4137–4142
Shi Z, Gu H (1997) Synth Commun 27:2789–2791
Shi Z, Gu H (1997) Synth Commun 27:2701–2707
Guo Y, Shi Z (2004) Synth Commun 34:3183–3189
Guo Y, Wu XL, Li JL, Xu RQ, Shi Z (2005) Synth Commun 35:2489–2494
Bai YJ, Lu J, Shi Z, Yang BQ (2001) Synlett 4:544–546
Li JL, Yin WT, Zhang J, Guo Y, Wu XL, Bai YJ, Shi Z (2008) Chem J Chinese 29:100–103
Becke AD (1993) J Chem Phys 98:1372–1377
Gill PMW, Johnson BG, Pople JA, Frisch MJ (1992) Int J Quantum Chem 44:319–331
Riemer-Sorensen S, Zioutas K, Hansen SH, Pedersen K, Dahle K, Liolios A (2007) Phys Rev Lett 8:1313011–1313014
Klontzas E, Mavrandonakis A, Tylianakis E, Froudakis GE (2008) Nano Lett 8:1572–1576
Zhang RQ, Wong NB, Lee ST, Zhu RS, Han KL (2000) Chem Phys Lett 319:213–219
Garrett ER, Gurkan T (1979) J Pharm Sci 68:26–32
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) GAUSSIAN03 Revision B.03. Gaussian Inc., Pittsburgh, PA
Binderbauer MW, Guo HY, Tuszewski M, Putvinski S, Sevier L, Barnes D (2010) Phys Rev Lett 105:0450031–0450034
Steckler R, Truhlar DG (1990) J Chem Phys 93:6570–6577
Liu YP, Lu DH, Lynch GC, Truong TN, Gonzalezlafont A, Truhlar DG (1993) Abstr Am Chem Soc 206:244
Schenter GK, Garrett BC, Truhlar DG (2003) J Chem Phys 119:5828–5833
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, H., Zhang, J., Wen, Z. et al. A theoretical study on the mechanism of a novel one-carbon unit transfer reaction. Struct Chem 22, 901–907 (2011). https://doi.org/10.1007/s11224-011-9776-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9776-1