Abstract
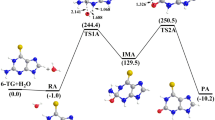
The hydrolytic deamination mechanism of adenosine to produce inosine was studied using density functional method on two models. One is adenine and the other is adenosine. Optimized geometries of reactants, intermediates, transition states, and products were determined at B3LYP/6-311G(d,p) level. IRC calculations were performed on the transition states to verify whether it is the real transition state that connects the corresponding intermediates. Single point calculations were carried out on the previous optimized geometries obtained during IRC calculations. Four pathways have been determined for the hydrolytic deamination of adenosine. Pathway d is the most favorable pathway. In this pathway a tetra-coordinated intermediate is formed through hydrolysis reaction, then the deamination reaction takes place, which causes the cleavage of C6–N10 bond and the creation of C=O bond. Unlike the deamination of adenine, the attacking side of water molecule has effect on the deamination of adenosine. The energy barriers of adenosine deamination are a little higher than those of adenine deamination.




Similar content being viewed by others
References
Glaser R, Rayat S, Lewis M, Son M-S, Meyer S (1999) J Am Chem Soc 121:6108–6119. doi:10.1021/ja9841254
Almatarneh MH, Flinn CG, Poirier RA, Sokalski WA (2006) J Phys Chem A 110:8227–8234. doi:10.1021/jp062300u
Labet V, Morell C, Grand A, Toro-Labbé A (2008) J Phys Chem A 112:11487–11494. doi:10.1021/jp8059097
Zhang A, Yang B, Li Z (2007) J Mol Struct THEOCHEM 819:95–101. doi:10.1016/j.theochem.2007.05.028
Almatarneh MH, Flinn CG, Poirier RA (2008) J Chem Inf Model 48:831–843. doi:10.1021/ci7003219
Becke AD (1993) J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Miehlich B, Savin A, Stoll H, Preuss H (1989) Chem Phys Lett 157:200–206. doi:10.1016/0009-2614(89)87234-3
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908–6918. doi:10.1021/jp048147q
Baboul AG, Curtiss LA, Redfern PC (1999) J Chem Phys 110:7650–7657. doi:10.1063/1.478676
Curtiss LA, Raghavachari K (1998) J Chem Phys 109:7764–7776. doi:10.1063/1.477422
Tang Y-Z, Sun J-Y, Sun H, Pan Y-R, Wang R-S (2008) Theor Chem Acc 119:297–303. doi:10.1007/s00214-007-0383-6
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskortz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision D.01. Gaussian Inc., Wallingford, CT
Matsubara T, Dupuis M, Aida M (2007) J Phys Chem B 111:9965–9974. doi:10.1021/jp072732k
Fuentes-Cabrera M, Sumpter BG, Šponer JE, Šponer J, Petit L, Wells JC (2007) J Phys Chem B 111:870–879. doi:10.1021/jp066465e
Liu H, Gauld JW (2008) J Phys Chem B 112:16874–16882
Close DM, Crespo-Hernández CE, Gorb L, Leszczynski J (2008) J Phys Chem A 112:12702–12706. doi:10.1021/jp807265y
Sousa SF, Fernandes PA, Ramos MJ (2007) J Phys Chem A 111:10439–10452. doi:10.1021/jp0734474
Labet V, Morell C, Cadet J, Eriksson LA, Grand A (2009) J Phys Chem A 113:2524–2533. doi:10.1021/jp808902j
Luo M, Schramm VL (2008) J Am Chem Soc 130:2649–2655. doi:10.1021/ja078008x
Acknowledgments
This work was supported by Scientific Research Reward Fund for Excellent Young and Middle-Aged Scientists of Shandong Province (Grant No. 2008BS02014) and Postdoctoral Science foundation of Shandong Province (Grant No. 200703077).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, C., Meng, F. Theoretical study on the hydrolytic deamination mechanism of adenosine. Struct Chem 20, 685–691 (2009). https://doi.org/10.1007/s11224-009-9461-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9461-9