Abstract
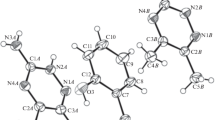
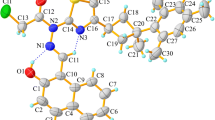
Crystals of the Schiff base derivative of gossypol with allylamine (GSBAL) were grown and subsequently examined by X-ray diffraction and FT-IR methods. The crystal space group is C2/c with a = 16.057(1) Å, b = 14.112(1) Å, c = 27.185(2) Å, β = 99.371(5)˚ and Z = 8. In the crystal, GSBAL exists in the enamine–enamine tautomeric form. The FT-IR spectral features of the crystals are in agreement with the X-ray data indicating that both parts of the molecule are similarly intramolecular hydrogen-bonded but different intermolecular hydrogen-bonded, although the molecule is symmetrically substituted. On the basis of the electrospray ionization mass spectrometry (ESI MS) experiments, it has been shown for the first time that Schiff base of gossypol forms complexes with the perchlorate anion and metal cations simultaneously. The ESI MS spectra of the 1:1:1 mixtures of GSBAL:GOS:M+, in the positive and negative ion detection mode, have indicated the preferential formation of the 1:1 complexes of GSBAL with M+ (Li, Na or K) and ClO4 − over the respective complexes forming between GOS and the metal cation or the anion. The PM5 semiempirical calculations have allowed visualization of the most energetically favourable structures of these two types of GSBAL complexes.












Similar content being viewed by others
References
Benedict CR, Liu J, Stipanovic RD (2006) Phytochemistry 67:356. doi:10.1016/j.phytochem.2005.11.015
Stipanovic RD, Puckhaber LS, Bell AA (2006) J Agric Food Chem 54:1633. doi:10.1021/jf052319e
Goldsmith KC, Hogarty MD (2005) Cancer Lett 228:133. doi:10.1016/j.canlet.2005.01.048
Xu L, Yang D, Wang S, Tang W, Liu M, Davis M, Chen J, Rae JM, Lawrence T, Lippman ME (2005) Mol Cancer Ther 4:197
Kovaci P (2003) Curr Med Chem 10:2711. doi:10.2174/0929867033456369
Olivier CL, Bauer JA, Woher KG (2005) Clin Cancer Res 11:5659
Dodou K (2005) Expert Opin Investig Drugs 14:1419. doi:10.1517/13543784.14.11.1419
Kenar JA (2006) J Am Oil Chem Soc 83:269. doi:10.1007/s11746-006-1203-1
Beketov KM, Talipov SA, Ibragimov BT, Praliev KD, Aripov TF (2003) KristallografiyaRuss 48:691
Przybylski P, Ratajczak-Sitarz M, Katrusiak A, Wojciechowski G, Schilf W, Brzezinski B (2003) J Mol Struct 655:293. doi:10.1016/S0022-2860(03)00319-3
Gdaniec M (1994) J Incl Phenom Macrocycl Chem 17:365. doi:10.1007/BF00707132
Talipov SA, Ibragimov BT, Izotova LYu, Tilyakov ZG, Dalimov DN (2004) Khim Prir Soedin (Russ.) 40:422
Przybylski P, Bejcar G, Huczyński A, Schroeder G, Brzezinski B, Bartl F (2006) Biopolymers 82:521. doi:10.1002/bip.20505
Dao V-T, Gaspard C, Mayer M, Werner GH, Nguyen SN, Michelot RJ (2000) Eur J Med Chem 35:805. doi:10.1016/S0223-5234(00)00165-3
Ziyaev KL, Kamaev FG, Baram NI, Biktimirov L, Ismailov AI (1997) Chem Nat Compd 33:545. doi:10.1007/BF02254801
Matlin SA, Roshdy S, Cass GB, Freitas CG, Longo RL, Malvestiti I (1990) J Braz Chem Soc 1:128
Dodou K, Anderson RJ, Lough WJ, Small DAP, Shelley MD, Groundwater PW (2005) Bioorg Med Chem 13:4228. doi:10.1016/j.bmc.2005.04.026
Przybylski P, Małuszyńska M, Brzezinski B (2005) J Mol Struct 750:152. doi:10.1016/j.molstruc.2005.04.027
Huczyński A, Stefańska J, Przybylski P, Brzezinski B, Bartl F (2008) Bioorg Med Chem Lett 18:2585. doi:10.1016/j.bmcl.2008.03.038
Przybylski P, Schilf W, Lewandowska W, Brzezinski B (2006) Biopolymers 83:213. doi:10.1002/bip.20548
Przybylski P, Brzezinski B, Bartl F (2004) Biopolymers 74:273. doi:10.1002/bip.20075
Przybylski P, Schroeder G, Brzezinski B (2004) J Mol Struct 699:65. doi:10.1016/j.molstruc.2004.03.056
Adams R, Morris RC, Geissman TA, Butterbaugh DJ, Kirkpatrick KC (1938) J Am Chem Soc 60:2193. doi:10.1021/ja01276a049
Sheldrick G (1986) SHELXS-86. Program for crystal structure solution, University of Göttingen
Sheldrick G (1997) SHELXL-97. Program for crystal structure refinement, University of Göttingen
Stewart JJP (2007) Cache Work System Pro Version 7.5.085 UserGuide, Fujitsu, Beaverton, Oregon, USA
Zubatyuk RI, Volovenko YM, Shishkin OV, Gorb L, Leszczynski J (2007) J Org Chem 72:725. doi:10.1021/jo0616411
Brzezinski B, Olejnik J, Paszc S, Aripov TF (1990) J Mol Struct 220:261. doi:10.1016/0022-2860(90)80116-2
Przybylski P, Brzezinski B (2002) Biopolymers (Biospectroscopy) 67:61. doi:10.1002/bip.10043
Sobczyk L, Grabowski SJ, Krygowski TM (2005) Chem Rev 105:3513. doi:10.1021/cr030083c
Przybylski P, Jasiński K, Brzezinski B, Bartl F (2002) J Mol Struct 611:193. doi:10.1016/S0022-2860(02)00075-3
Acknowledgement
Financial assistance of the Polish Ministry of Science and Higher Education–Grant No. N204 056 32/1432 is gratefully acknowledged by P. Przybylski.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Przybylski, P., Pyta, K., Ratajczak-Sitarz, M. et al. X-ray, FT-IR, ESI MS and PM5 studies of Schiff base of gossypol with allylamine and its complexes with alkali metal cations and perchlorate anion. Struct Chem 19, 983–995 (2008). https://doi.org/10.1007/s11224-008-9385-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-008-9385-9