Abstract
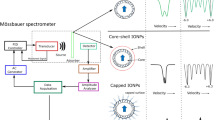
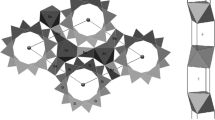
The chemical reactions between iron(III) and indole-3-acetic (IAA), -propionic (IPA), and -butyric (IBA) acids were studied in acidic aqueous solutions. The motivation of this work was that IAA is one of the most powerful natural plant-growth-regulating substances (phytohormones of the auxin series). Mössbauer spectra of the frozen aqueous solutions of iron(III) with indole-3-alkanoic acids as ligands (L), showed parallel reactions between Fe3+ and the ligands. Partly, it resulted in a complex formation which precipitated in aqueous solution and partly, in a redox process with iron(II) and the oxidised indole-3-alkanoic acids as products. The Mössbauer parameters of the Fe2+ species suggested a hexaaquo coordination environment. The chemical composition and coordination structure of the precipitated complexes were investigated using elemental analysis, Mössbauer spectroscopy, Fourier transform infrared (FTIR) and Raman spectroscopic techniques. The complexes were soluble in some organic solvents. So, Mössbauer, FTIR and solution X-ray diffraction measurements were carried out on the solution of complexes in acetone, hexadeutero acetone and methanol, respectively. The data obtained supported the existence of the μ-dihydroxo-bridging structure of the dimer: [L2Fe<(OH)2>FeL2] (where L is indole-3-propionate, -acetate or -butyrate).















Similar content being viewed by others
References
Marumo S (1986) In: Takahashi N (ed) Chemistry of plant hormones. CRC Press, Inc., Boca Raton, Florida (USA), Chapter 2, pp 9–56
Weyers JDB, Paterson NW (2001) New Phytologist 152:375–407
Woodward AW, Bartel B (2005) Ann Bot (London) 95:707–735
Lambrecht M, Okon Y, Vande Broek A, Vanderleyden J (2000) Trends Microbiol 8:298–300
Costacurta A, Vanderleyden J (1995) Crit Rev Microbiol 21:1–18
Kamnev AA, Kuzmann E (1997) Biochem Mol Biol Int 41:575–581
Kamnev AA, Kuzmann E (1997) In: Carmona P, Navarro R, Hernanz A, (eds) Spectroscopy of biological molecules: Modern trends. Annex. UNED Press, Madrid, pp 85–86
Kamnev AA, Shchelochkov AG, Perfiliev YuD, Tarantilis PA, Polissiou MG (2001) J Mol Struct 563–564, 565–572
Kamnev AA, Kuzmann E, Perfiliev YuD, Vankó Gy, Vértes A (1999) J Mol Struct 482–483, 703–711
Kovács K, Kamnev AA, Shchelochkov AG, Kuzmann E, Medzihradszky-Schweiger H, Mink J, Vértes A (2004) J Radioanal Nucl Chem 262:151–156
Kamnev AA, Kuzmann E, Perfiliev YuD, Vértes A, Ristić M, Popović S, Musić S (2000) J Radioanal Nucl Chem 246:123–129
Gazaryan IG, Lagrimini LM, Ashby GA, Thorneley RNF (1996) Biochem J 313:841–847
Hinman RL, Lang J (1965) Biochemistry (USA) 4:144–158
Harrod JF, Guerin C (1979) Inorg Chim Acta 37:141–144
Vértes A, Parak F (1971) J Chem Soc Dalton Trans 2062–2068
Klencsár Z, Kuzmann E, Vértes A (1996) J Radioanal Nucl Chem 210:105–114
Radnai T, Ohtaki H (1996) Mol Phys 87:103–121
Hajdu F, Pálinkás G (1972) J Appl Cryst 5:395–401
Levy HA, Danford MD, Narten AH (1966) Oak Ridge National Laboratory Rep., Nr. 3960, 54 pp
Hajdu F (1972) Acta Cryst A28:250–252
Pálinkás G, Radnai T (1976) Acta Cryst A32:666–668
Krogh-Moe K (1956) Acta Cryst 2:951–953
Cromer DT, Waber JT (1965) Acta Cryst 18:104–109
International Tables for X-ray Crystallography (1974) vol 4, The Kynoch Press, Birmingham (UK), pp 366
Vértes A, Nagy DL (eds) (1990) Mössbauer spectroscopy of frozen solutions, Akad Kiadó, Budapest
Ferraro JR, Walker WR (1965) Inorg. Chem, 4:1382–1386
Degen A, Bolte M (2001) Acta Cryst. E – Structure Reports Online, 57, Part 11, O999-O1000
Narten AH, Habenschuss A (1984) J Chem Phys 80:3387–3391
Acknowledgements
The authors are grateful to Professor G. Pálinkás (Budapest, Hungary), Dr. P. A. Tarantilis (Athens, Greece) and Dr. A. G. Shchelochkov (Saratov, Russia) for many stimulating discussions and to L. Hajba for recording the far-infrared spectra.
This work was supported in parts by The Hungarian Science Foundation (OTKA Grant T43687), NATO (Expert Visit Grants LST.EV.980141 and CBP.NR.NREV.981748; Collaborative Linkage Grants LST.CLG.977664 and LST.NR.CLG.981092), Russian Academy of Sciences’ Commission (Grant No. 205 under the 6th Competition-Expertise of research projects), as well as under the Agreements on Scientific Cooperation between the Russian and Hungarian Academies of Sciences for 2002–2004 and 2005–2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kovács, K., Kamnev, A.A., Mink, J. et al. Mössbauer, vibrational spectroscopic and solution X-ray diffraction studies of the structure of iron(III) complexes formed with indole-3-alkanoic acids in acidic aqueous solutions. Struct Chem 17, 105–120 (2006). https://doi.org/10.1007/s11224-006-9005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-006-9005-5