Abstract
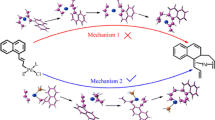
Hydroallylation of norbornadiene (NBD) with allyl formate (AF) in the presence of the Pd0 complexes with the formation of 5-allyl-2-norbornene was modeled by the method based on the DFT-PBE/L11 density functional theory. According to the calculation results, 5-allyl-2-norbornene is formed via two mechanisms. The first assumes that the C-C bond between the NBD and allyl ligand is formed before the cleavage of the formyl C-H bond and elimination of CO2, whereas following the second mechanism the bond is formed after these processes. For both mechanisms, Pd(AF)(MeCN) is the catalytically active complex and CNBD-CAll bond formation is the rate-determining step with the Gibbs activation energy equal to 22.8 and 21.3 kcal mol−1for the first and second mechanisms, respectively. High selectivity to 5-allyl-2-norbornene in the absence of phosphine ligands is attributable to the kinetically hindered formation of the second C-C bond needed for the generation of product of oxidative allylation of NBD. The predominance of the exo-substituted product is due to the formation of the thermodynamically stable complex with the bidentate coordination of NBD favored by the endo-coordination of the NBD molecule.
Similar content being viewed by others
References
V. R. Flid, M. L. Gringolts, R. S. Shamsiev, E. Sh. Finkelshtein, Russ. Chem. Rev., 2018, 87, 1169.
M. Catellani, G. Chiusoli, E. Dradi, G. Salerno, J. Organomet. Chem., 1979, 177, 29.
U. M. Dzhemilev, R. I. Khusnutdinov, G. A. Tolstikov, Russ. Chem. Rev., 1987, 56, 36.
U. M. Dzhemilev, R. I. Khusnutdinov, D. K. Galeev, O. M. Nefedov, G. A. Tolstikov, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1987, 36, 122.
E. M. Evstigneeva, R. S. Shamsiev, V. R. Flid, Vestn. MITKhT im. M. V. Lomonosova [Bulletin of M. V. Lomonosov Moscow Institute of Chemical Technologies], 2006, 1, No. 3, 3 (in Russian).
E. M. Evstigneeva, V. R. Flid, Russ. Chem. Bull., 2008, 57, 837.
I. P. Stolyarov, A. E. Gekhman, I. I. Moiseev, A. Yu. Kolesnikov, E. M. Evstigneeva, V. R. Flid, Russ. Chem. Bull., 2007, 56, 320.
S. A. Durakov, R. S. Shamsiev, V. R. Flid, A. E. Gekhman, Russ. Chem. Bull., 2018, 67, 2234.
S. A. Durakov, R. S. Shamsiev, V. R. Flid, A. E. Gekhman, Kinet. Catal., 2019, 60, 245.
D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151.
D. N. Laikov, Yu. A. Ustynyuk, Russ. Chem. Bull., 2005, 54, 820.
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865.
D. N. Laikov, Chem. Phys. Lett., 2005, 416, 116.
R. S. Shamsiev, V. R. Flid, Russ. Chem. Bull., 2013, 62, 2301.
D. V. Dmitriev, R. S. Shamsiev, Ha Ngok Thien, V. R. Flid, Russ. Chem. Bull., 2013, 62, 2385.
K. T. Egiazaryan, R. S. Shamsiev, V. R. Flid, Fine Chem. Technol., 2019, 14, No. 6, 56.
E. M. Simmons, J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066.
Z. Mao, C. T. Campbell, ACS Catal., 2020, 10, 4181.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the Russian Science Foundation (Project No. 18-13-00415). The calculations were performed using computational resources of the Joint Supercomputer Center of the Russian Academy of Sciences.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 2, pp. 316–322, February, 2021.
Rights and permissions
About this article
Cite this article
Shamsiev, R.S., Egiazaryan, K.T. & Flid, V.R. Modeling of the mechanism of reductive allylation of norbornadiene in the presence of Pd0 complexes. Russ Chem Bull 70, 316–322 (2021). https://doi.org/10.1007/s11172-021-3087-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3087-5