Abstract
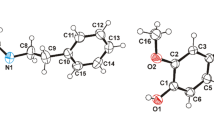
Derivatives of the amino acids sarcosine and proline – triflates TsN(Me)CH2C(O)N(Me)-CH2SiMe2OTf and Ns–Pro–N(Me)CH2SiMe2OTf (Ns is 4-NO2C6H4SO2) – were synthesized by the reaction of trimethylsilyl triflate with halosilanes prepared previously. Bromide NsNHCH(Me)C(O)N(Me)CH2SiMe2Br was synthesized by the cleavage of the Si–O–Si moiety of the appropriate disiloxane with excess acetyl bromide. The X-ray diffraction study of these compounds and of the previously characterized bromide TsN(Ac)CH2C(O)N(Me) CH2SiMe2Br showed that the Si–O coordination bond lengths in the triflates are 1.7692(14)– 1.8623(14) Å. The interatomic distances between the bromine and silicon atoms are 2.7095(8) and 2.9704(7) Å, which indicates that these bonds are weak. To elucidate the nature of Si…X bonding (X = OTf, Br), the topological analysis of electron density was performed and 29Si NMR chemical shifts were calculated. The interatomic Si…X interaction in triflate TsN(Me)CH2C(O)- N(Me)CH2SiMe2OTf and bromide TsN(Ac)CH2C(O)N(Me)CH2SiMe2Br is weak. In triflate Ns–Pro–N(Me)CH2SiMe2OTf and bromide NsNHCH(Me)C(O)N(Me) CH2 SiMe2Br, the Si…X interaction is significantly stronger and corresponds to a weak coordination bond. Hence, the tosylates can be considered as salts, whereas the para-nitrobenzenesulfonyl derivatives are monochelated five-coordinate silicon complexes.
Similar content being viewed by others
References
A. A. Macharashvili, V. E. Shklover, Yu. T. Struchkov, G. I. Oleneva, E. P. Kramarova, A. G. Shipov, Y. I. Baukov, J. Chem. Soc., Chem. Commun., 1988, 683–685.
Y. E. Ovchinnikov, A. A. Macharashvili, Yu. T. Struchkov, A. G. Shipov, Y. I. Baukov, J. Struct. Chem., 1994, 35, 91–100.
A. R. Bassindale, M. Borbaruah, S. J. Glynn, D. J. Parker, P. G. Taylor, J. Organomet. Chem., 2000, 606, 125–131.
A. Bassindale, J. Organomet. Chem., 2001, 619, 132–140.
A. R. Bassindale, M. Borbaruah, S. J. Glynn, D. J. Parker, P. G. Taylor, J. Chem. Soc., Perkin Trans. 2, 1999, 2099–2109.
A. R. Bassindale, Y. I. Baukov, M. Borbaruah, S. J. Glynn, V. V. Negrebetsky, D. J. Parker, P. G. Taylor, R. Turtle, J. Organomet. Chem., 2003, 669, 154–163.
A. V. Vologzhanina, A. A. Korlyukov, M. Y. Antipin, Acta Cryst., 2008, B64, 448–455.
E. F. Belogolova, V. F. Sidorkin, Russ. Chem. Bull., 2002, 51, 1472–1476.
E. F. Belogolova, E. P. Doronina, M. A. Belogolov, V. F. Sidorkin, J. Mol. Struct.: THEOCHEM, 2010, 950, 72–78.
V. F. Sidorkin, E. F. Belogolova, V. A. Pestunovich, J. Mol. Struct.: THEOCHEM, 2001, 538, 59–65.
A. A. Nikolin, E. P. Kramarova, A. A. Korlyukov, D. E. Arkhipov, A. G. Shipov, Yu. I. Baukov, A. A. Lagunin, T. A. Shmigol, Vad. V. Negrebetsky, Russ. Chem. Bull., 2017, 66, 571–573.
A. A. Korlyukov, K. A. Lyssenko, M. Yu. Antipin, Russ. Chem. Bull., 2002, 51, 1423–1432.
A. A. Macharashvili, V. E. Shklover, Y. T. Struchkov, Yu. I. Baukov, E. P. Kramarova, G. I. Oleneva, J. Organomet. Chem., 1987, 327, 167–172.
S. Muhammad, A. R. Bassindale, P. G. Taylor, L. Male, S. J. Coles, M. B. Hursthouse, Organometallics, 2011, 30, 564–571.
M. Sohail, R. Panisch, A. Bowden, A. R. Bassindale, P. G. Taylor, A. A. Korlyukov, D. E. Arkhipov, L. Male, S. Callear, S. J. Coles, M. B. Hursthouse, R. W. Harrington, W. Clegg, Dalton Trans., 2013, 42, 10971–10981.
A. R. Bassindale, D. J. Parker, P. G. Taylor, N. Auner, B. Herrschaft, J. Organomet. Chem., 2003, 667, 66–72.
A. A. Korlyukov, S. A. Pogozhikh, Yu. E. Ovchinnikov, K. A. Lyssenko, M. Yu. Antipin, A. G. Shipov, O. A. Zamyshlyaeva, E. P. Kramarova, V. V. Negrebetsky, I. P. Yakovlev, Yu. I. Baukov, J. Organomet. Chem., 2006, 691, 3962–3975.
A. G. Shipov, E. P. Kramarova, H. Fang, D. E. Arkhipov, A. A. Nikolin, S. Y. Bylikin, V. V. Negrebetsky, A. A. Korlyukov, N. A. Voronina, A. R. Bassindale, P. G. Taylor, Yu. I. Baukov, J. Organomet. Chem., 2013, 741–742, 114–121.
N. F. Lazareva, A. Yu. Nikonov, Russ. Chem. Bull., 2017, 66, 1138–1162.
F. H. Allen, Acta Cryst, 2002, B58, 380–388.
N. K. Hansen, P. Coppens, Acta Cryst., 1978, A34, 909–921.
A. A. Korlyukov, Russ. Chem. Rev., 2015, 84, 422–440.
R. F. W. Bader, Atoms in Molecules. A Quantum Theory, Clarendon Press, Oxford, 1990.
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19.
E. Espinosa, I. Alkorta, I. Rozas, J. Elguero, E. Molins, Chem. Phys. Lett., 2001, 336, 457–461.
M. G. Voronkov, V. P. Mileshkevich, Yu. A. Yuzhelevskii, Siloksanovaya svyaz´ [Siloxane Bonding], Nauka, Novosibirsk, 1976 (in Russian).
E. L. Kupche, E. Ya. Lukevits, Russ. Chem. Rev., 1989, 58, 1777.
V. V. Negrebetsky, V. V. Negrebetsky, A. G. Shipov, E. P. Kramorova, Y. I. Baukov, J. Organomet. Chem., 1995, 496, 103–107.
V. V. Negrebetsky, S. N. Tandura, Yu. I. Baukov, Russ. Chem. Rev., 2009, 78, 21–51.
L. Olsson, C.-H. Ottosson, D. Cremer, J. Am. Chem. Soc., 1995, 117, 7460–7479.
E. P. Doronina, V. F. Sidorkin, N. F. Lazareva, J. Phys. Chem. A, 2015, 119, 3663–3673.
E. F. Belogolova, V. F. Sidorkin, J. Phys. Chem. A, 2013, 117, 5365–5376.
A. A. Nikolin, O. V. Kuznetsova, D. E. Arkhipov, E. P. Kramarova, A. G. Shipov, A. N. Egorochkin, A. A. Korlyukov, Yu. I. Baukov, Vad. V. Negrebetskii, Russ. Chem. Bull., 2013, 62, 1892–1899.
A. A. Nikolin, E. P. Kramarova, A. G. Shipov, Y. I. Baukov, V. V. Negrebetsky, A. A. Korlyukov, D. E. Arkhipov, A. Bowden, S. Y. Bylikin, A. R. Bassindale, P. G. Taylor, Organometallics, 2012, 31, 4988–4997.
. A. Nikolin, V. V. Negrebetsky, Russ. Chem. Rev., 2014, 83, 848–883.
The Chemistry of Organic Silicon Compounds, Eds S. Patai, Z. Rappoport, Y. Apeloig, Wiley, Chichester–New York, 1989.
A. G. Shipov, A. A. Korlyukov, E. P. Kramarova, D. E. Arkhipov, S. Yu. Bylikin, Kh. Fan, S. A. Pogozhikh, T. P. Murasheva, V. V. Negrebetsky, V. N. Khrustalev, Yu. E. Ovchinnikov, A. R. Bassindale, P. G. Taylor, Yu. I. Baukov, Russ. J. Org. Chem., 2011, 81, 2428–2439.
A. K. Wolf, J. Glinnemann, L. Fink, E. Alig, M. Bolte, M. U. Schmidt, Acta Cryst., 2010, B66, 229–236.
A. G. Shipov, E. P. Kramarova, T. P. Murasheva, A. A. Korlyukov, S. A. Pogozhikh, S. A. Tarasenko, V. V. Negrebetsky, I. P. Yakovlev, Yu. I. Baukov, Russ. J. Org. Chem., 2011, 81, 2428–2439.
E. Klieger, E. Schroder, Arch. Pharm. (Weinheim), 1973, 306, 834.
A. A. Nikolin, D. E. Arkhipov, A. G. Shipov, E. P. Kramarova, N. A. Koval´chuk, A. A. Korlyukov, V. V. Negrebetsky, Yu. I. Baukov, A. R. Bassindale, P. G. Taylor, A. Bowden, S. Yu. Bylikin, Chem. Heterocycl. Compd., 2011, 47, 1565–1583.
G. M. Sheldrick, Acta Cryst., 2008, A64, 112–122.
G. M. Sheldrick, Acta Cryst, 2015, C71, 3–8.
G. Kresse, J. Hafner, Phys. Rev. B, 1993, 47, 558.
G. Kresse, J. Hafner, Phys. Rev. B, 1994, 49, 14251–14269.
G. Kresse, J. Furthmuller, Phys. Rev. B, 1996, 54, 11169.
G. Kresse, J. Furthmuller, Comput. Mat. Sci., 1996, 6, 15–50.
G. Kresse, D. Joubert, Phys. Rev. B, 1999, 59, 1758.
X. Gonze, J.-M. Beuken, R. Caracas, F. Detraux, M. Fuchs, G.-M. Rignanese, L. Sindic, M. Verstraete, G. Zerah, F. Jollet, M. Torrent, A. Roy, M. Mikami, P. Ghosez, J.-Y. Raty, D. C. Allan, Comput. Mat. Sci., 2002, 25, 478–492.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, J. A. Pople, Gaussian 03, Revision C.01; Gaussian, Inc.: Wallingford, 2004.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 0137–0148, January, 2019.
Rights and permissions
About this article
Cite this article
Korlyukov, A.A., Arkhipov, D.E., Volodin, A.D. et al. Mono-C,O-chelated bromo- and triflatosilanes with an amino acid moiety: salts or covalently bonded complexes?. Russ Chem Bull 68, 137–148 (2019). https://doi.org/10.1007/s11172-019-2429-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-019-2429-z