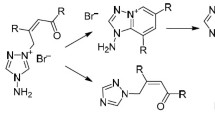
Abstract
Methods for the preparation of stable 1,4,6,10-tetraazaadamantane quaternary derivatives were developed based on quaternization of a tertiary nitrogen atom in tris(β-oximinoalkyl)amines or isomeric 4,6,10-trihydroxy-1,4,6,10-tetraazaadamantanes. This process constitutes a convenient approach to the introduction of a hydrophilic 1,4,6,10-tetraazaadamantane moiety into lipophilic molecules in order to increase their solubility in water. 4,6,10-Trihydroxy-1,4,6,10-tetraazaadamantane N-oxide was synthesized by the oxidation of the tertiary nitrogen atom in the tris-oxime and subsequent intramolecular cyclotrimerization of the oximino groups. Quantum chemical calculations showed that the quaternization of the annular nitrogen atom led to a considerable stabilization of tetraazaadamantane framework as compared to the open-chain form of tris-oxime.
Similar content being viewed by others
References
G. A. Mansoori, P. L. B. de Araujo, E. S. de Araujo, Diamondoid Molecules: With Applications in Biomedicine, Materials Science, Nanotechnology and Petroleum Science, World Scientific, Singapore, 2012
H. Schwertfeger, A. A. Fokin, P. R. Schreiner, Angew. Chem., Int. Ed., 2008, 47, 1022
L. Wanka, K. Iqbal, P. R. Schreiner, Chem. Rev., 2013, 113, 3516
A. I. Kuznetsov, Azaadamantany, Uspekhi khimii adamantana [Azaadamantanes, Advances in Adamantane Chemistry], Khimiya, Moscow, 2007 (in Russian).
M. Grillaud, J. Russier, A. Bianco, J. Am. Chem. Soc., 2014, 136, 810
A. M. Balija, R. E. Kohman, S. C. Zimmerman, Angew. Chem., Int. Ed., 2008, 47, 8072
R. E. Kohman, S. C. Zimmerman, Chem. Commun., 2009, 794
K. Nasr, N. Pannier, J. V. Frangioni, W. Maison, J. Org. Chem., 2008, 73, 1056
C. Fleck, E. Franzmann, D. Claes, A. Rickert, W. Maison, Synthesis, 2013, 45, 1452.
A. J. Kessel, Anti-Infect. Agents, 2013, 11, 1
A. T. Nielsen, A. P. Chafin, S. L. Christian, D. W. Moore, M. P. Nadler, R. A. Nissan, D. J. Vanderah, Tetrahedron, 1998, 54, 11793
A. A. Fokin, T. S. Zhuk, A. E. Pashenko, P. O. Dral, P. A. Guchenko, J. E. P. Dahl, R. M. K. Carlson, T. V. Koso, M. Serafin, P. R. Schreiner, Org. Lett., 2009, 11, 3068
I. S. Golovanov, A. Yu. Sukhorukov, Yu. V. Nelyubina, Yu. A. Khomutova, S. L. Ioffe, V. A. Tartakovsky, J. Org. Chem., 2015, 80, 6728.
A. Butlerow, Ann. Chem. Pharm., 1859, 111, 242
A. Butlerow, Justus Liebigs Ann. Chem., 1859, 111, 250.
A. N. Semakin, A. Yu. Sukhorukov, A. V. Lesiv, S. L. Ioffe, K. A. Lyssenko, Y. V. Nelyubina, V. A. Tartakovsky, Org. Lett., 2009, 11, 4072
A. N. Semakin, A. Yu. Sukhorukov, S. L. Ioffe, V. A. Tartakovsky, Synthesis, 2012, 1095.
A. N. Semakin, A. Yu. Sukhorukov, Yu. V. Nelyubina, Yu. A. Khomutova, S. L. Ioffe, V. A. Tartakovsky, J. Org. Chem., 2014, 79, 6079.
G. Matthaiopoulos, Chem. Ber., 1898, 31, 2396.
M. Meldal, C. W. Tornøe, Chem. Rev., 2008, 108, 2952
K. B. Sharpless, R. Manetsch, Expert Opin. Drug Discov., 2006, 1, 525
S. Hassan, T. J. J. Müller, Adv. Synth. Catal., 2015, 357, 617
A. N. Semakin, D. P. Agababyan, S. Kim, S. Lee, A. Yu. Sukhorukov, K. G. Fedina, J. Oh, S. L. Ioffe, Tetrahedron Lett., 2015, 56, 6335.
G. N. Grover, J. Lam, T. H. Nguyen, T. Segura, H. D. Maynard, Biomacromolecules, 2012, 13, 3013
S. Ulrich, D. Boturyn, A. Marra, O. Renaudet, P. Dumy, Chem. Eur. J., 2014, 20, 34.
A. Anderson, A. C. Boyd, A. Byford, A. C. Campbell, D. K. Gemmell, N. M. Hamilton, D. R. Hill, C. Hill-Venning, J. J. Lambert, M. S. Maidment, V. May, R. J. Marshall, J. A. Peters, D. C. Rees, D. Stevenson, H. Sundaram, J. Med. Chem., 1997, 40, 1668
J. Sear, British J. Anaesthesia, 1997, 79, 417
F. J. Cespedes-Guirao, A. B. Ropero, E. Font-Sanchis, Angel Nadal, F. Fernandez-Lazaro, A. Sastre-Santos, Chem. Commun., 2011, 47, 8307.
A. J. Kirby, I. V. Komarov, N. Feeder, J. Am. Chem. Soc., 1998, 120, 7101
G. Fraenkel, D. Watson, J. Am. Chem. Soc., 1975, 97, 231.
L. Wen, M. Li, J. B. Schlenoff, J. Am. Chem. Soc., 1997, 119, 7726.
A. Maruani, S. Alom, P. Canavelli, M. T. W. Lee, R. E. Morgan, V. Chudasama, S. Caddick, Chem. Commun., 2015, 51, 5279.
Author information
Authors and Affiliations
Corresponding author
Additional information
Based on the materials of the International Congress on the Heterocyclic Chemistry "KOST-2015" (October 18—23, 2015, Moscow, Russia).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 2270—2277, September, 2016.
Rights and permissions
About this article
Cite this article
Semakin, A.N., Golovanov, I.S., Sukhorukov, A.Y. et al. Synthesis of 1,4,6,10-tetraazaadamantane quaternary derivatives. Russ Chem Bull 65, 2270–2277 (2016). https://doi.org/10.1007/s11172-016-1577-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1577-7