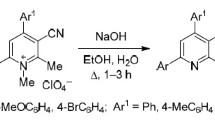
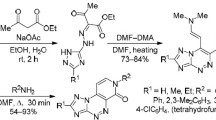
Quaternization of 4-amino-4H-1,2,4-triazole with γ-bromodipnones and 5-(bromomethyl)-2,2,6,6-tetramethylhept-4-en-3-one occurred at the N-1 nitrogen atom, with the formation of quaternary salts that were cyclized in the presence of bases into [1,2,4]triazolo[1,5-a]-pyridine derivatives. Quaternary 4-amino-4H-1,2,4-triazolium salts and aromatic 1-amino-1H-[1,2,4]triazolo[1,5-a]pyridinium salts can be deaminated by treatment with nitrous acid, giving 1-substituted 1,2,4-triazoles and the respective [1,2,4]triazolo[1,5-a]pyridines.
Similar content being viewed by others
References
(a) Schulze, V.; Mais, F. J. US Patent 2015148542. (b) Ishimoto, K.; Fukuda, N.; Nagata, T.; Sawai, Y.; Ikemoto, T. Org. Process Res. Dev. 2014, 18, 122. (c) Schirok, H.; Schulze, V.; Kosemund, D.; Briem, H.; Bader, B.; Bomer, U.; Wengner, A. M.; Siemeister, G.; Lienau, P. WO Patent 2014195274. (d) Flohr, A.; Groebke, Z. K.; Koerner, M. WO Patent 2013041472. (e) Flohr, A.; Gobbi, L.; Groebke, Z. K.; Koerner, M.; Peters, J.-U. WO Patent 2012000970. (f) Fantasia, S. M.; Puentener, K. WO Patent 2014177493. (g) Middlemiss, D.; Leriche, C. WO Patent 2011161159. (h) Cartwrite, M. W.; Edwards, C.; Goodacre, S. C.; Patel, S.; Ray, N. C.; Sajad, M.; Yuen, P.; Zak, M. E.; Cheng, X. WO Patent 2015032286. (i) Miller, T. A.; Petrocchi, A.; Romeo, E. T.; Vitharana, D.; Kattar, S. D.; Northrup, A. B.; Altman, M. D.; Anthony, N. J. WO Patent 2015095444. (j) Ellard, K.; Ramsden, N. WO Patent 2012000970. (k) Crawford, J. J.; Ortwine, D. F.; Young, W. B. US Patent 20130116262.
Jones, G. In Advances in Heterocyclic Chemistry; Katritzky, A. R., Ed.; Elsevier: New York, 2002, Vol. 83, p. 1.
(a) Zribi, L.; Zribi, F.; Marco-Contelles, J.; Chabchoub, F.; Ismaili, Lh. Synth. Commun. 2017, 47, 1934. (b) Matsuda, Y.; Katou, K.; Motokawa, C.; Uemura, T. Heterocycles 2000, 53, 213. (c) Mishchuk, A.; Shtil, N.; Poberezhnyk, M.; Nazarenko, K.; Savchenko, T.; Tolmachev, A. Tetrahedron Lett. 2016, 57, 1056.
(a) Glover, E. E.; Rowbottom, K. T. J. Chem. Soc., Perkin Trans. 1 1976, 367. (b) Mammoliti, O; Quinton, E. M.; Loones, K. T. J.; Nguyen, A. T.; Wouters, J.; Lommen, G. V. Tetrahedron 2013, 69, 1669. (c) Bhatt, A.; Singh R. K.; Kant, R. Chem. Heterocycl. Compd. 2018, 54, 1111. [Khim. Geterotsikl. Soedin. 2018, 54, 1111.] (d) Lv, J.; He, Z.; Zhang, J.; Guo, Y.; Han, Z.; Bao, X. Tetrahedron 2018, 74, 3996. (e) Hoerer, S.; Heine, N. Bioorg. Med. Chem. Lett. 2018, 26, 3227. (f) Ribeiro, C. J. A.; Kankanala, J.; Xie, J.; Williams, J.; Aihara, H.; Wang, Z. Bioorg. Med. Chem. Lett. 2019, 29, 257.
Kovtunenko, V.; Potikha, L.; Turov, A. Synth. Commun. 2004, 34, 3609.
Holm, S. C., Straub, B. F. Org. Prep. Proced. Int. 2011, 43, 319.
Potikha, L. M.; Turelyk, A. R.; Kovtunenko, V. A.; Turov, A. V. Chem. Heterocycl. Compd. 2010, 46, 82. [Khim. Geterotsikl. Soedin. 2010, 95.]
Potikha, L. M.; Turelyk, A. R.; Kovtunenko, V. A.; Turov, A. V. Chem. Heterocycl. Compd. 2010, 46, 223. [Khim. Geterotsikl. Soedin. 2010, 275.]
Daily, L. A.; Miller, K. M. J. Org. Chem. 2013, 78, 4196.
Gilchrist, T. L. In Advances in Heterocyclic Chemistry; Katritzky, A. R., Ed.; Elsevier: New York, 1987, Vol. 41, p. 41.
(a) Wassermann, H. H.; Aubrey, N. E. J. Am. Chem. Soc. 1953, 75, 96. (b) Potikha, L. M.; Turelyk, A. R.; Kovtunenko, V. A. Chem. Heterocycl. Compd. 2009, 45, 1184. [Khim. Geterotsikl. Soedin. 2009, 1478.] (с) van Tamalen, E. E.; Whitesides, T. H. J. Am. Chem. Soc. 1968, 90, 3894.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(4/5), 367–373
Electronic supplementary material
ESM 1
(PDF 836 kb)
Rights and permissions
About this article
Cite this article
Kovtunenko, V.A., Potikha, L.M., Shelepyuk, A.V. et al. A new method for the construction of [1,2,4]triazolo[1,5-a]pyridine system. Chem Heterocycl Comp 55, 367–373 (2019). https://doi.org/10.1007/s10593-019-02466-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02466-w