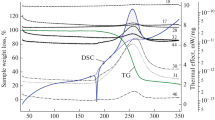
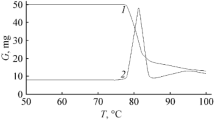
Abstract
The decomposition of mono-, di-, and trinitropyrazole derivatives in the condensed state was studied by the manometric method. The reaction rate depends on the number and position of nitro groups in the pyrazole cycle and on the polarity of the medium and aggregate state of the substance. The activation energy of the initial non-catalytic stage of decomposition Е 1 decreases on going from monoto trinitropyrazoles from 142 to 132 kJ mol−1, and the preexponential factor is 109±0.5 s−1. In a diphenyl solution the decomposition rate is lower than that in the melt, and this difference decreases with increasing in the number of nitro groups in the molecule. For the decomposition of trinitropyrazole in the solid state, Е 1 decreases by 10 kJ mol−1. All these facts are explained in terms of the mechanism, according to which the reaction occurs as the oxidation of the adjacent carbon atom by the nitro group and proceeds via a strongly polar cyclic transition state.
Similar content being viewed by others
References
I. L. Dalinger, G. P. Popova, I. A. Vatsadze, T. K. Shkineva, S. A. Shevelev, Russ. Chem. Bull. (Int. Ed.), 2009, 58, 2185 [Izv. Akad. Nauk, Ser. Khim., 2009, 2120]
I. L. Dalinger, I. A. Vatsadze, T. K. Shkineva, G. P. Popova, S. A. Shevelev, Mendeleev Commun., 2010, 20, 253.
G. Hervé, C. Roussel, H. Graindorge, Angew. Chem., Int. Ed. Engl., 2010, 49, 3177.
Yu. V. Nelyubina, I. L. Dalinger, K. A. Lyssenko, Angew. Chem., Int. Ed. Engl., 2011, 50, 2892.
Y. Zhang, Y. Guo, T.-H. Joo, D. A. Parrish, J. M. Shreeve, Chem. Eur. J., 2010, 16, 10778.
Y. Zhang, D. A. Parrish, J. M. Shreeve, J. Mater. Chem., 2012, 22, 12659.
J. Zhong, C. He, D. A. Parrish, J. M. Shreeve, Chem. Eur. J., 2013, 19, 8929.
C. He, J. Zhang, D. A. Parrish, J. M. Shreeve, J. Mater. Chem. A, 2013, 1, 2863.
T. M. Klapötke, A. Penger, C. Pfluger, J. Strierstorfer, M. Suceska, Eur. J. Inorg. Chem., 2013, 4667
Y. Zhang, D. A. Parrish, J. M. Shreeve, J. Mater. Chem. A, 2014, 2, 3200.
S. Ek, L. Yudina Wahlström, N. Latypov, J. Chem. Eng., 2011, 5, 929.
A. A. Zaytsev, I. L. Dalinger, S. A. Chevelev, Russ. Chem. Rev., 2009, 78, 589.
I. L. Dalinger, I. A. Vatsadze, T. K. Shkineva, G. P. Popova, B. I. Ugrak, S. A. Shevelev, Russ. Chem. Bull. (Int. Ed.), 2010, 59, 1631 [Izv. Akad. Nauk, Ser. Khim., 2010, 1589]
I. L. Dalinger, T. K. Shkineva, I. A. Vatsadze, G. P. Popova, S. A. Shevelev, Mendeleev Commun., 2011, 21, 48.
A. B. Sheremetev, Y. L. Yudin, N. V. Palysaeva, K. Y. Suponitsky, J. Heterocycl. Chem., 2012, 49, 394.
N. V. Palysaeva, K. P. Kumpan, M. I. Struchkova, I. L. Dalinger, A. V. Kormanov, N. S. Aleksandrova, V. M. Chernyshev, D. F. Pyreu, K. Yu. Suponitsky, A. B. Sheremetev, Org. Lett., 2014, 16, 406.
I. Dalinger, S. Shevelev, V. Korolev, D. Khakimov, T. Pivina, A. Pivkina, O. Ordzhonikidze, Yu. Frolov, J. Therm. Anal. Calorim., 2011, 105, 509.
I. Dalinger, A. Pivkina, O. Gryzlova, V. Korolev, T. Pivina, Yu. Nelyubina, S. Shevelev, Yu. Frolov, Proc. 38th Intern. Pyrotechnics Seminar (Denver, Colorado, USA, 12–15 June 2012), Denver, 2012, 479.
P. Ravi, G. M. Gore, A. K. Sikder, S. P. Tewari, Thermochim. Acta, 2012, 528, 53.
Y. L. Wang, F. Q. Zhao, Y. P. Ji, Q. Pan, J. H. Yi, T. An, W. Wang, T. Yu, X. M. Lu, J. Anal. Appl. Pyrolysis, 2012, 98, 231.
P. Ravi, A. A. Vargeese, S. P. Tewari, Thermochim. Acta, 2012, 550, 83.
J. W. A. M. Janssen, H. J. Koeners, C. G. Kruse, C. L. Habraken, J. Org. Chem., 1973, 38, 1777.
Yu. Shu, V. V. Dubikhin, G. M. Nazin, Khim. Fiz. [Chemical Physics], 2010, 29, 29.(in Russian).
G. B. Manelis, G. M. Nazin, V. G. Prokudin, Russ. Chem. Bull. (Int. Ed.), 2011, 60, 1440 [Izv. Akad. Nauk, Ser. Khim., 2011, 1417].
G. M. Khrapkovskii, A. G. Shamov, E. V. Nikolaeva, D. V. Chachkov, Russ. Chem. Rev., 2009, 78, 903.
N. H. Kinstle, I. G. Stam, J. Org. Chem., 1970, 35, 1771.
G. B. Manelis, G. M. Nazin, Yu. I. Rubtsov, V. A. Strunin, Termicheskoe razlozhenie i gorenie vzryvchatykh veshchestv i porokhov [Thermal Decomposition and Combustion of Explosives and Gunpowders], Nauka, Moscow, 1996, 223 pp. (in Russian).
D. C. Nonhhebel, J. C. Walton, Free-Radical Chemistry, Cambridge University Press, Cambridge, 1974.
A. Streitwieser, Molecular Orbital Theory for Organic Chemists, Wiley, New York, 1961, 489 pp.
S. W. Benson, Thermochemical Kinetics, J. Wiley and Sons INC, New York–London–Sydney, 1963, 320 pp.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 0126–0131, January, 2015.
Rights and permissions
About this article
Cite this article
Dubikhin, V.V., Nazin, G.M., Prokudin, V.G. et al. Kinetics and mechanism of thermal decomposition of nitropyrazoles. Russ Chem Bull 64, 127–131 (2015). https://doi.org/10.1007/s11172-015-0830-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-015-0830-9