Abstract
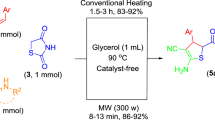
A rapid, efficient, and reliable catalyst-free procedure for the synthesis of quinoxaline derivatives in glycerol under focused microwave irradiation was developed. The reaction proceeded in glycerol without any catalyst making this methodology valuable from both economic and environmental viewpoints.
Similar content being viewed by others
References
N. R. Candeias, L. C. Branco, P. M. P. Gois, C. A. M. Afonso, A. F. Trindade, Chem. Rev., 2009, 109, 2703
M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol, P. Machado, Chem. Rev., 2009, 109, 4140
P. J. Walsh, H. Li, C. A. De Parrodi, Chem. Rev., 2007, 107, 2503.
C. J. Li, Chem. Rev., 1993, 93, 2023
U. M. Lindstrom, Chem. Rev., 2002, 102, 2751
J.-P. Genet, M. Savignac, J. Organomet. Chem., 1999, 576, 305
D. Sinou, Adv. Synth. Catal., 2002, 344, 221
K. Manabe, S. Kobayashi, Chem. Eur. J., 2002, 8, 4094
C. J. Li, Chem. Rev., 2005, 105, 3095
D. Dallinger, C. O. Kappe, Chem. Rev., 2007, 107, 2563
B. H. Lipshutz, S. Ghorai, Aldrichim. Acta, 2008, 41, 59
A. Chanda, V. V. Folkin, Chem. Rev., 2009, 109, 725.
J. Chen, S. K. Spear, J. G. Huddlestom, R. D. Rogers, Green Chem., 2005, 7, 64
N. R. Candeias, L. C. Branco, P. M. Gois, C. A. M. Afonso, A. F. Trindade, Chem. Rev., 2009, 109, 2703
L. Walter, Nature, 2003, 423, 930
K. H. Lam, L. Xu, L. Feng, Q.-H. Fan, F. L. Lam, W.-H. Lo, A. S. C. Chan, Adv. Synth. Catal., 2005, 347, 1755
J. Mao, J. Guo, F. Fang, S. Ji, Tetrahedron, 2008, 64, 3905
S. Chandrasekhar, C. Narsihmulu, S. S. Sultana, N. R. Reddy, Org. Lett., 2002, 4, 4399
W.-J. Zhou, K.-H. Wang, J.-X. Wang, Adv. Synth. Catal., 2009, 351, 1378
V. V. Namboodiri, R. S. Varma, Green Chem., 2001, 3, 146
L. Xu, W. Chen, J. Ross, J. Xiao, Org. Lett., 2001, 3, 295
L. Gao, W. Mai, Synlett, 2006, 2553
W.-B. Wang, S.-M. Lu, P.-Y. Yang, X.-W. Han, Y.-G. Zhou, J. Am. Chem. Soc., 2003, 125, 10536.
M. Pagliaro, M. Rossi, The Future of Glycerol: New Usages for a Versatile Raw Material, RSC Publ., Cambridge, 2008, 104 pp.
Y. Gu, F. Jerome, Green Chem., 2010, 12, 1127
A. E. Diaz-Alvarez, J. Francos, B. Lastra-Barreira, P. Crochet, V. Cadierno, Chem. Commun., 2011, 47, 6208
Y. Gu, J. Barrault, F. Jerome, Adv. Synth. Catal., 2008, 350, 2007
H. M. Bachhav, S. B. Bhagat, V. N. Telvekar, Tetrahedron Lett., 2011, 43, 5697.
P. W. Erhardt, J. Med. Chem., 1987, 30, 231
B. E. Tomczuk, C. R. Taylor, Jr., L. M. Moses, D. B. Sutherland, Y. S. Lo, D. N. Johnson, W. B. Kinnier, B. F. Kilpatrick, J. Med. Chem., 1991, 34, 2993
A. Y. Chen, C. Yu, B. Gatto, L. F. Liu, Proc. Natl. Acad. Sci. USA, 1993, 90, 8131
T. Roth, M. L. Morningstar, P. L. Boyer, S. H. Hughes, R. W. Buckheit, C. J. Michejda, Jr., J. Med. Chem., 1997, 40, 4199
L. E. Seitz, W. J. Suling, R. C. Reynolds, J. Med. Chem., 2002, 45, 5604.
C. Venkatesh, B. Singh, P. K. Mahata, H. Ila, H. Junjappa, Org. Lett., 2005, 7, 2169
R. S. Robinson, R. J. K. Taylor, Synlett, 2005, 1003
S. A. Raw, C. D. Wilfred, R. J. K. Taylor, Org. Biomol. Chem., 2004, 788
M. M. Heravi, K. Bakhtiari, M. H. Tehrani, N. M. Javadi, H. A. Oskooie, ARKIVOC, 2006, Part 16, 16
H. R. Darabi, S. Mohandessi, K. Aghapoor, F. Mohsenzadeh, Catal. Commun., 2007, 389
S. V. More, M. N. V. Sastry, C.-C. Wang, C.-F. Yao, Tetrahedron Lett., 2005, 46, 6345.
S. A. Raw, C. D. Wilfred, R. J. K. Taylor, Chem. Commun., 2003, 2286.
S. V. More, M. N. V. Sastry, C.-F. Yao, Green Chem., 2006, 8, 91.
C. Srinivas, C. N. S. S. P. Kumar, V. J. Rao, S. Palaniappan, J. Mol. Catal. A: Chem., 2007, 265, 227.
M. M. Heravi, S. Taheri, K. Bakhtiari, H. A. Oskooie, Catal. Commun., 2007, 8, 211.
S. Sithambaram, Y. Ding, W. Li, X. Shen, F. Gaenzler, S. L. Suib, Green Chem., 2008, 9, 1029.
D. Fang, K. Gong, Z. Fei, X. Zhou, Z. Liu, Catal. Commun., 2008, 9, 317.
M. Larhed, G. Lindeberg, A. Hallberg, Tetrahedron Lett., 1996, 37, 8219
M. Larhed, A. Hallberg, J. Org. Chem., 1996, 61, 9582
S. Caddick, R. Fitzmaurice, Tetrahedron, 2009, 65, 3325
H. A. Oskooie, M. M. Heravi, N. Nami, A. Nazari, Heterocycl. Commun., 2005, 11, 101.
P. Appukkuttan, V. P. Mehta, P. Van der Eycken, Chem. Soc. Rev., 2010, 39, 1467
V. Polshettiwar, R. S. Varma, Green Chem., 2010, 12, 743
P. Appukkuttan, E. Van der Eycken, Eur. J. Org. Chem., 2008, 1133
Y. Hemasri, Heterocycl. Commun., 2009, 15, 423.
C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250
C. O. Kappe, E. Van der Eycken, Chem. Soc. Rev., 2010, 39, 1280.
J. M. Kremsner, C. O. Kappe, J. Org. Chem., 2006, 71, 4651
J.-F. Zhou, G.-X. Gong, L.-T. An, Y. Liu, F.-X. Zhu, Y.-L. Zhu, S.-J. Ji, Synlett, 2008, 3163
C. Henry, A. Haupt, S. C. Turner, J. Org. Chem., 2009, 74, 1932
L. Bai, J.-X. Wang, Adv. Synth. Catal., 2008, 350, 315.
W.-J. Zhou, K.-H. Wang, J.-X. Wang, J. Org. Chem., 2009, 74, 5599
W.-J. Zhou, K.-H. Wang, J.-X. Wang, D.-F. Huang, Eur. J. Org. Chem., 2010, 416.
J.-X. Wang, Y. Xi, X. Wu, Y. Hu, Z. Du, Synth. Commun., 1998, 28, 4619.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 1244–1247, May, 2013.
Rights and permissions
About this article
Cite this article
Zhou, W.J., Zhang, X.Z., Sun, X.B. et al. Microwave-assisted synthesis of quinoxaline derivatives using glycerol as a green solvent. Russ Chem Bull 62, 1244–1247 (2013). https://doi.org/10.1007/s11172-013-0171-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-013-0171-5